chemistry u3 aos2 (chemical equlibrium)
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
supernatant liquid
a clear, liquid, free of precipitate, that forms during the chemical process of precipitation
open system VS closed system
open system
matter and energy can be exchanged with surroundings
closed system
only energy can be exchanged with surroundings
equilibrium being reached?
rate of forward reaction = rate of reverse reaction
concentration of reactants/ products remains/ not change
** ‘dynamic’ equlibrium
how do we have reverse reactions?
when the product molecules collide with sufficient energy to break their bonds, that larger than or equals to the Ea of the reverse reaction, they can reform reactants
what are we most interested about in a chemical reaction?
stoichiometry
rate
extent
characteristics of a chemical equilibrium
both forward and reverse reactions occur simultaneously (represented by ⇌)
rate of forward = rate of reverse
constant concentration of both reactants and products
dynamic equilibrium
→ concentrations not change, but both reactions are still occurring
reactions do not go to completion; extent of reactions varies (i.e. amount of reactants and products are NOT the same)
what does both Q and K stand for? what is their equation?
Q = reaction quotient
K = equilibrium constant
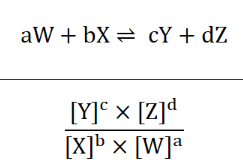
what does K measure?
it measures the extent of reaction AND equilibrium yield
how would the reaction equation (e.g. coef/ reversed) affect the equilibrium expression and its units?
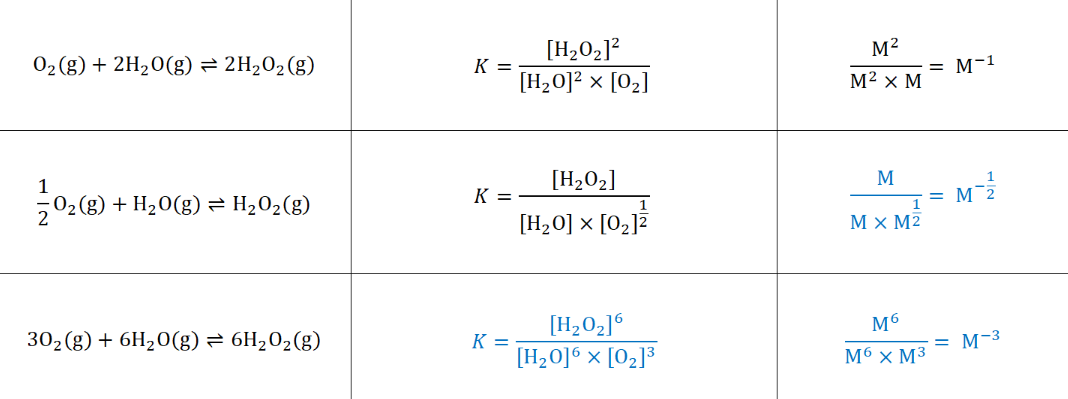
what does Q</ =/ >K mean
Q<K
more forward reaction is needed to produce more products to reach equilibrium
Q=K
equilibrium is reached
Q>K
more reverse reaction is needed to convert the products back into reactants to reach equilibrium
what is a homogeneous equilibrium
the states of reactants and products are the same
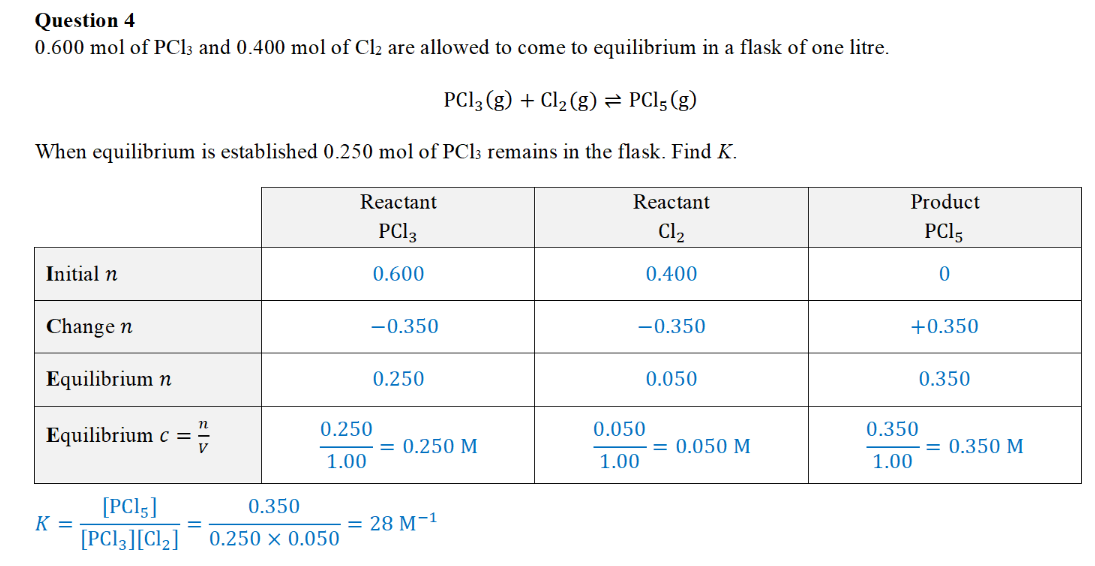
ICE table
** initial n for products is usually 0 (unless stated)
considerations for concentration graph
horizontal section (indicates at equilibrium as the concentrations are no longer changing)
all species reach equilibrium at the same time
the changes in curved part are dependent on the mole ratio
the changes in the straight part are dependent on the original volume
factors that alter the equilibrium position
** changing equilibrium position ≠ equilibrium constant K
concentration
pressure
temperature
state the LCP
according to LCP, if the system in equilibrium is subjected to a change, the position of equilibrium will shift to partially oppose the change
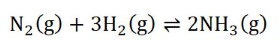
how would increase in H2 change the concentration graph
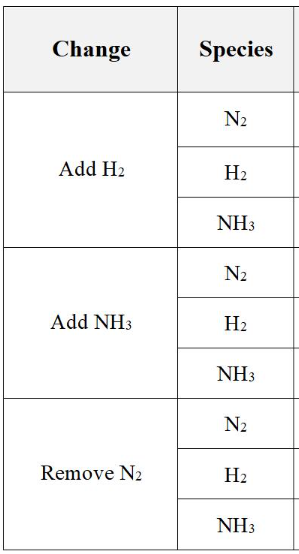
describe the effect of each of the changes to the overall concentration
what is the effect of dilution on the concentration graph?
immediate decrease in concentration (straight line decrease; which the size is relative to the original conc)
→ e.g. volume is doubled → conc will be reduced by half
→ mole ratio has no effect yet coz the reaction has not moved forward/ back
partial oppose (curved part) after the immediate decrease in concentration to reach equilibrium again
why don’t the coefficient from the reaction equation have any impact on the concentration of the species immediately after dilution?
because the immediately after dilution, the reaction has not shifted yet, therefore the mole ratio is irrelevant
(only the initial concentration of species are relevant at this stage)
what is the effect of increase in pressure (decrease in volume) on the concentration graph?
immediate increase in concentration (straight line increase; which the size is relative to the original volume)
→ e.g. volume is reduced by half → conc will be increase by twice
→ mole ratio has no effect yet coz the reaction has not moved forward/ back
** tbh doesn’t matter how long the straight line part is, as long as all the straight line changes are proportional
partial oppose (curved part) after the immediate decrease in concentration to reach equilibrium again
changes that DOES NOT affect the equilibrium position
the number of moles in reactants = number of moles in products (because the system cannot shift forward/ backwards to change the total moles present)
inert gas is added which does not involve a change in volume (because only the total pressure of gases in the system will increase; but not the pressure of individual gas component
catalyst (because it increases the rate of both reactions equally)
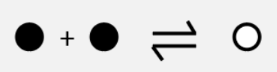
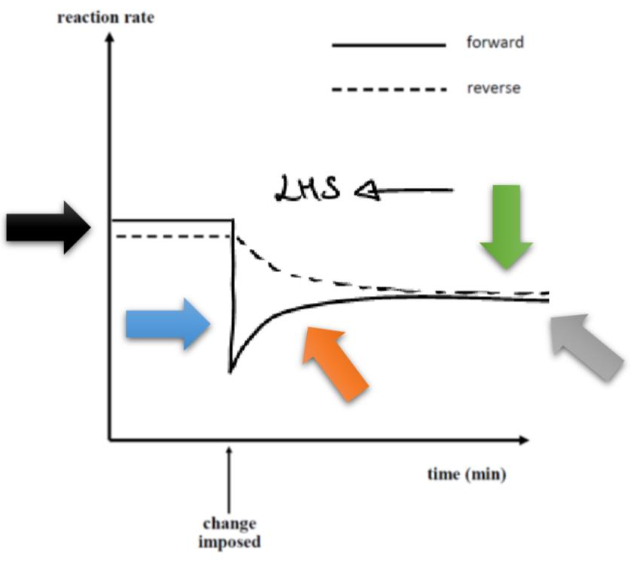
how would an increase in pressure affect the rate of both reactions at different stages? (before the change/ when pressure is first increased/ after the partial oppose)
(before the change)
both forward and reverse reactions are in the same rate
(when pressure is increased)
both forward and reverse reactions are faster
forward reaction has a higher rate than reverse reaction (given more particles in reactants)
→ ‘forward reaction is favoured’/ ‘reaction is shifted towards the right)
(after the partial oppose/ equilibrium re-established)
both forward and reverse reactions are faster
both forward and reverse reactions are the same rate
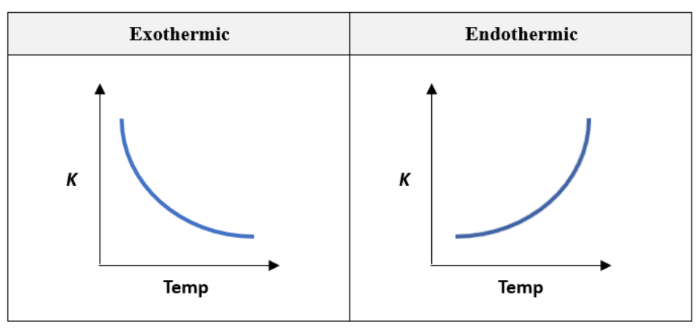
graphs of the effect of increasing temperature on K for both exothermic and endothermic reaction
** graphs are NON-LINEAR
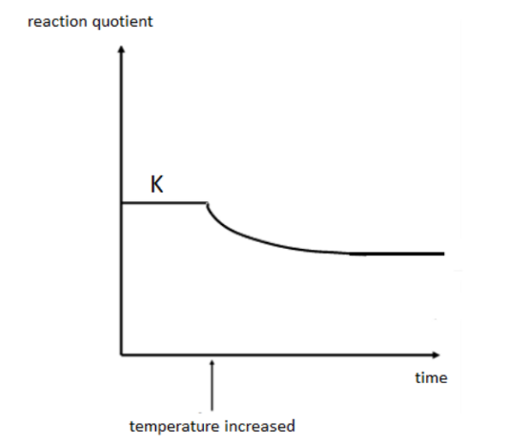
how would a graph (of Q against time) look like when temperature is increased in an exothermic reaction?
how would you describe the effect of a temperature change on the changes in concentration graph? (gradual/ instant)
gradual change, no instant change therefore no (vertical) straight lines
where would the economic dilemma occur? exothermic/ endothermic reactions?
exothermic
increase in temperature, increase the rate of reaction, however decreases the yield (less products formed)
decrease in temperature, increases the yield (more products), however decreases the rate of reaction
endothermic
increase in temperature, increases both the reaction rate and the yield
effect if each changed condition on the shift in equilibrium (cover the answer)
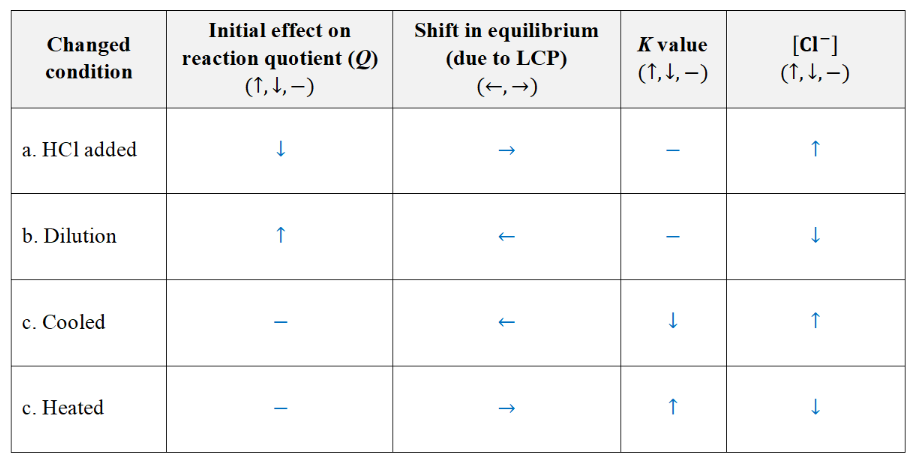
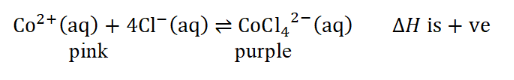
how would a graph (of Q against time) look like for dilution?
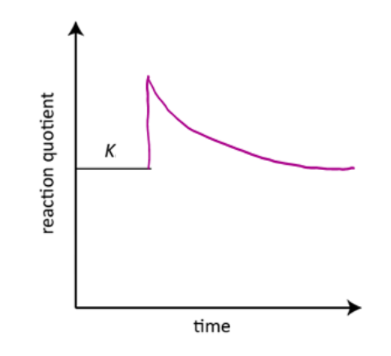
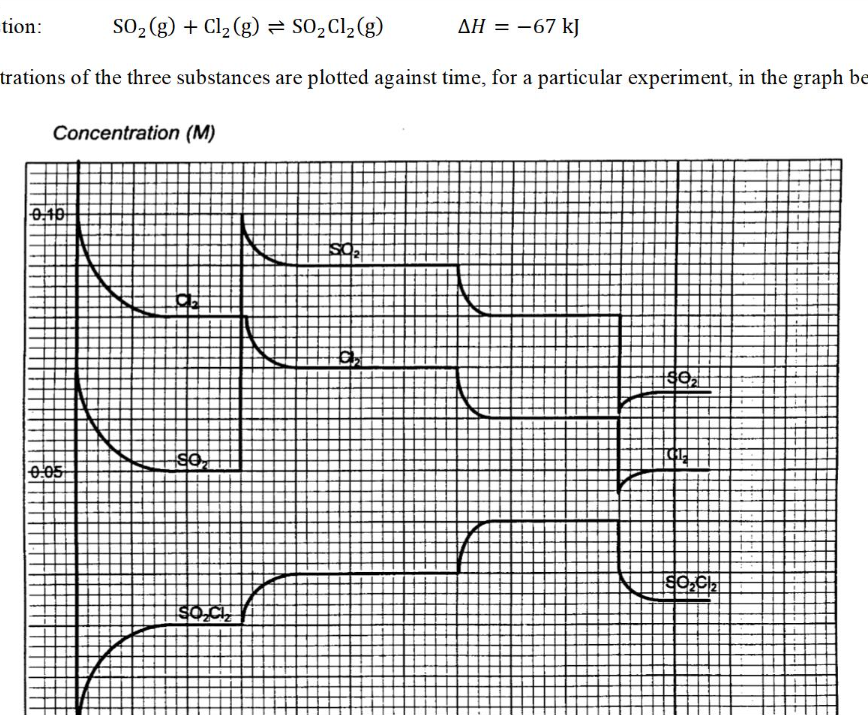
identify the event occurred at the fourth curve (the third change) when all the concentrations decreased immediately
increase in volume/ decrease in pressure
** CANNOT be dilution (all the species are in gas state!!)
when will a concentration graph look the same as a mole against time graph?
when the volume does not change
conflicts for fast rate and high equilibrium yields (compare the conditions for fast rate and for high equilibrium yield)
(for fast rate)
high temperature
high concentration/ pressure
high surface area
addition of catalyst
(for high equilibrium yield)
high temperature for endo; low temperature for exo
pressure depends on the relative mole ratio for reactants and products
more reactants
removal of products
how would a removal of reactants affect the rate of reaction compare to the original rates of both
removal of reactants decreases the rate of both reactions (lower rates), as fewer particles present
reverse reaction will then be favoured to increase moles of reactants
therefore the rate of forward reaction will start to increase again
however the overall reaction rates of both will still be lower than the original reaction rates