Organic Chemistry (Princeton Review)
1/60
Earn XP
Description and Tags
MCAT Organic chemistry. Reading the book front to back and adding terms to this deck
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
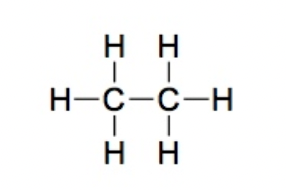
Alkane
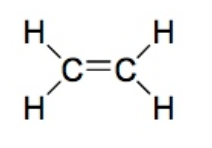
Alkene
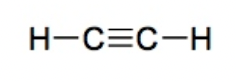
Alkyne
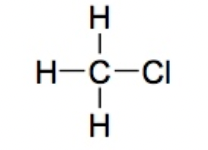
Alkyl halide
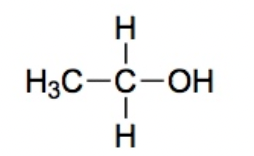
Alcohol
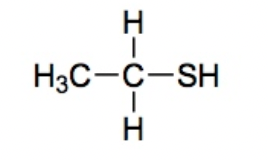
Thiol
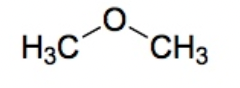
Ether
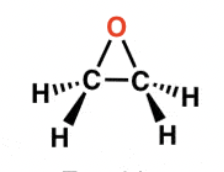
Epoxide
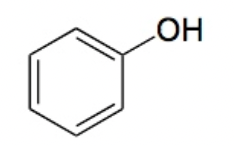
Phenol
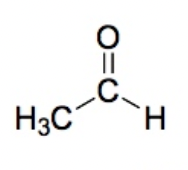
Aldehyde
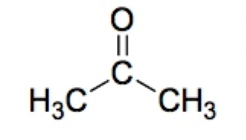
Ketone
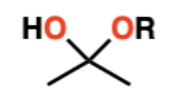
Hemiacetal
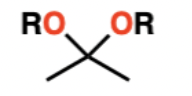
Acetal
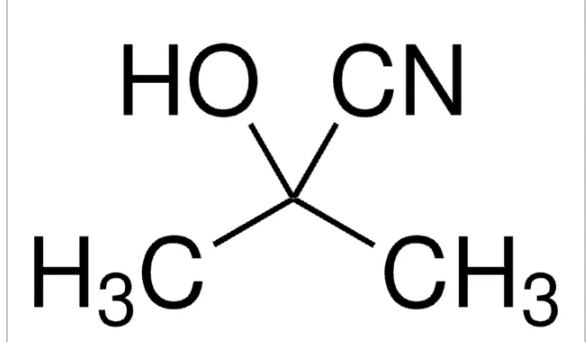
Cyanohydrin
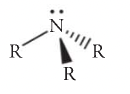
Amine
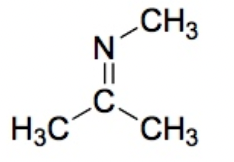
Imine
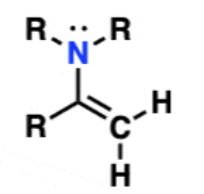
Enamine
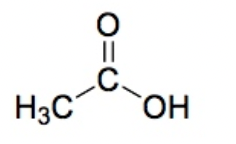
Carboxylic Acid
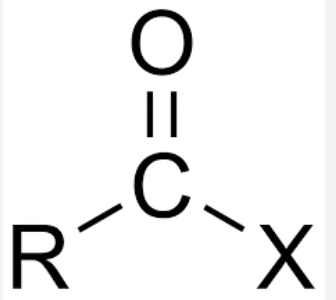
Acid Halide
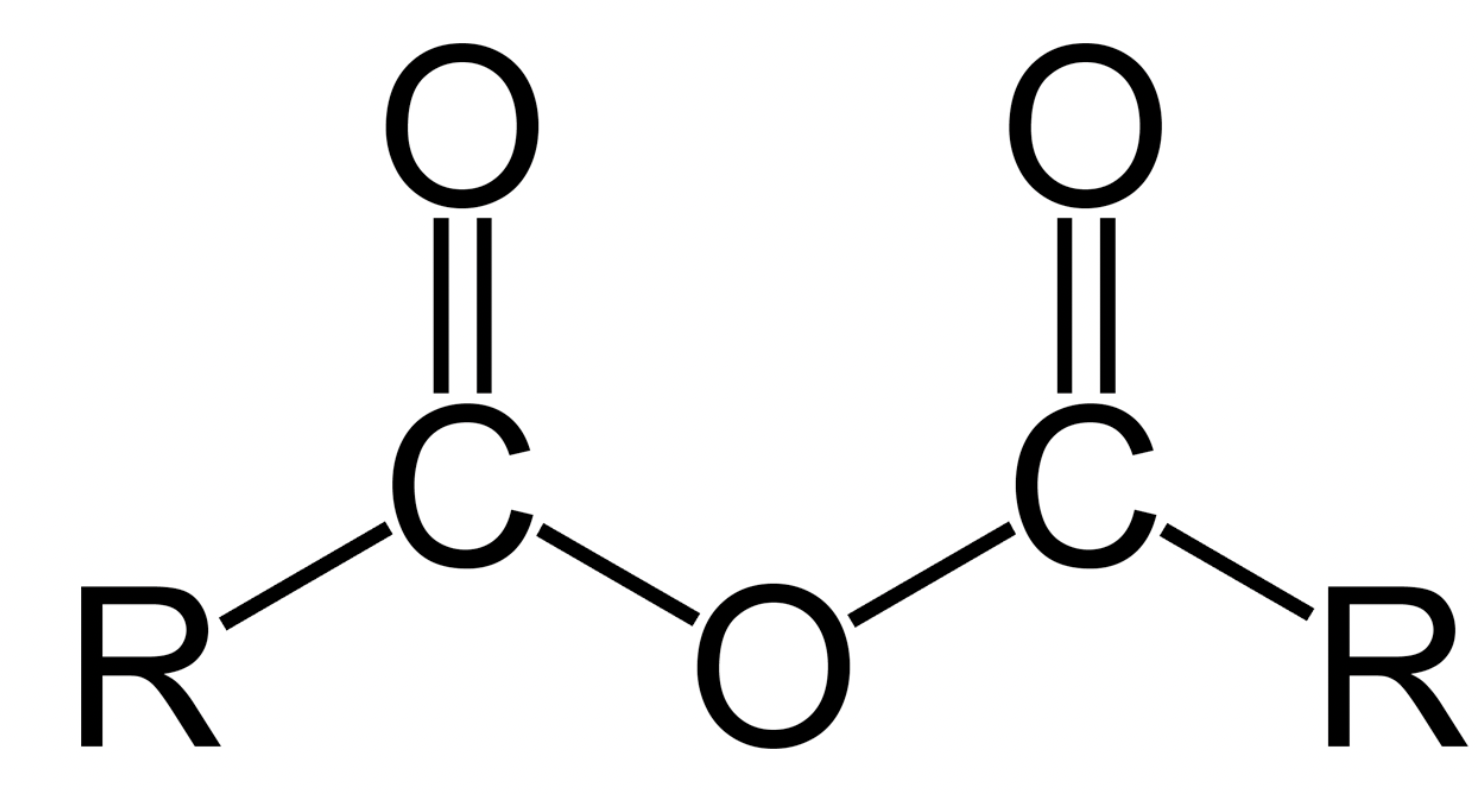
Acid Anhydride
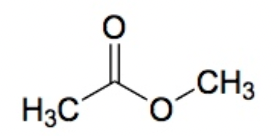
Ester
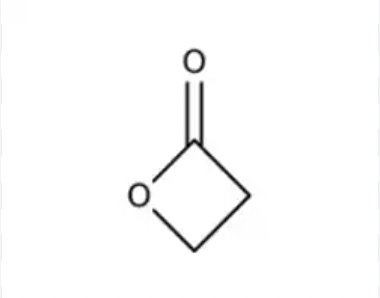
Lactone
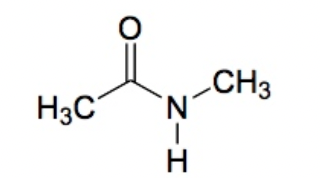
Amide
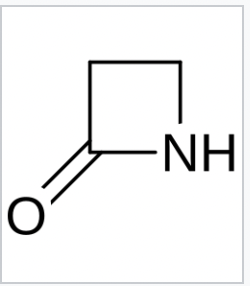
Lacatm

Methyl
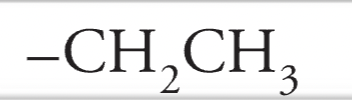
Ethyl

Propyl
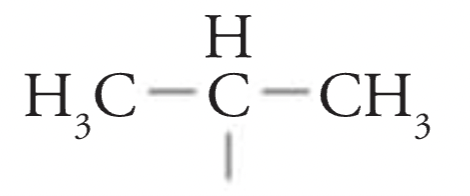
Isopropyl
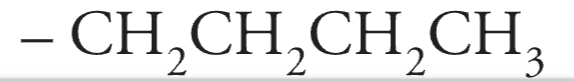
Butyl
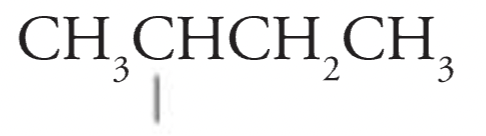
sec-Butyl
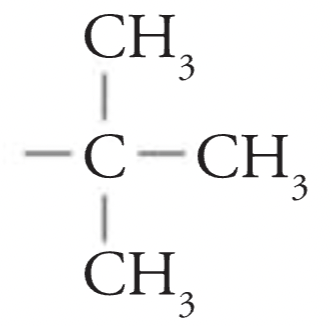
tert-Butyl
What is the purpose of extraction in lab techniques?
To separate one substance from a mixture by adding a solvent in which the wanted compound is soluble
What happens in a liquid-liquid extraction?
A compound is placed in a solution and mixed with an immiscible second solvent, the mixture is then separated into two phases
What is the distribution coefficient?
The ratio of a substances solubility in two different solvents
What does Thin Layer Chromatography do?
Separates compounds based on polarity
In TLC, how does polarity affect movement on the plate?
The more polar = the slower it travels
The less polar = the quicker it travels
What is the Rf value in TLC?
The distance traveled by an individual component divided by the distance traveled by the solvent
What does Column Chromatography separate?
Used to separate bulky compounds by polarity
In Column Chromatography, how does polarity affect elution?
The more polar = the slower it is to elute
The less polar = the faster it is to elute
What does Ion Exchange Chromatography separate based on?
Separate ions based on charge, commonly separates proteins
In Cation Exchange, which ions leave first?
Positive ions leave first, negative ions remain in the column
In Anion Exchange, which ions leave first?
Negative ions leave first, positive ions remain in the column
What is High Performance Liquid Chromatography (HPLC)?
An advance version of all chromatography techniques.
In HPLC how does polarity affect elutions?
The more polar = the quicker to elute
The less polar = the slower to elute
What does Size Exclusion Chromatography separate based on?
Separates based on size
In Size Exclusion Chromatography, which molecules elute fast/slow?
Larger molecules = elute faster
Smaller molecules = elute slower
What is Affinity Chromatography used for?
To purify proteins or nucleic acids based off specific interactions
What is an Affinity Tag in Affinity Chromatography
A small molecular tag added to the N- or C- terminus of a protein
What does Gas Chromatography separate?
The separation of compounds based on different volatiles
In Gas Chromatography, how does volatility affect elutioh?
The more volatile = faster elution
The less volatile = slower elution
In separation techniques, what is the purpose of distillation?
A technique to raise the temp of a liquid till it overcomes intermolecular forces, then condensing the vapor back into liquid separating based on boiling points
What is simple distillation used for?
Removing trace impurities from a pure compound or separating compounds with large differences in boiling points
What is Fractional Distillation used for?
Separating mixtures with a small difference in boiling points