Misc
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
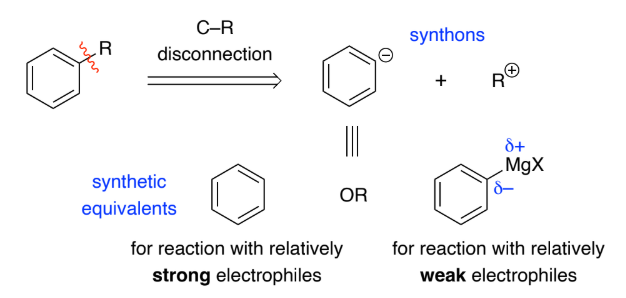
Normally the bond to the aromatic ring is broken to give a synthon with a _________ charge on the aromatic ring as they react with __________ in ____________substitutions
negative, electrophiles, electrophilic
OH & OR groups are difficult to add to a benzene ring, so don’t disconnect them but use _______ as a starting material
phenol
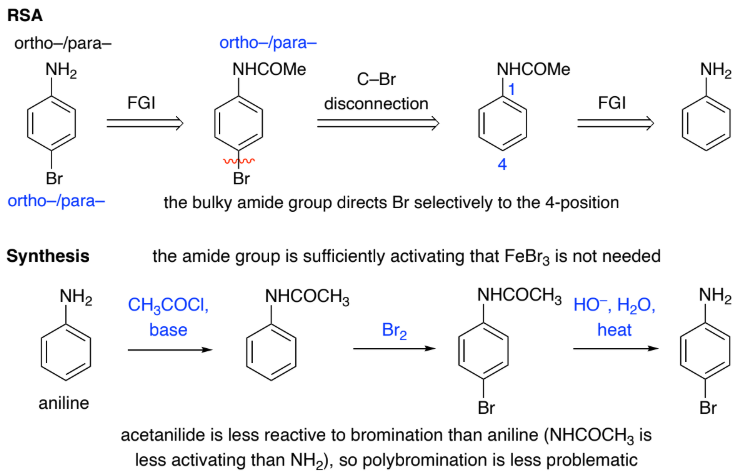
To improve regioselectivity, introduce a temporary _______ group e.g.
convert an NH2 group into a larger NHCOCH3 group
blocking
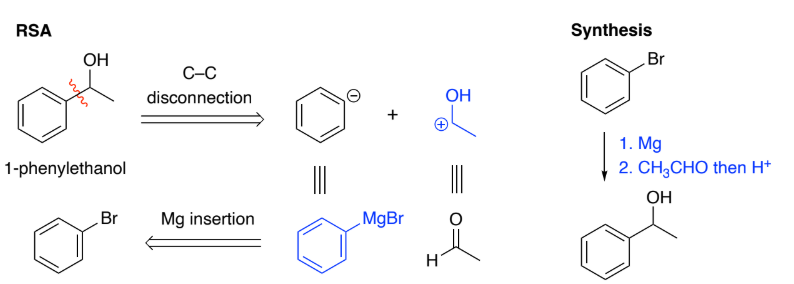
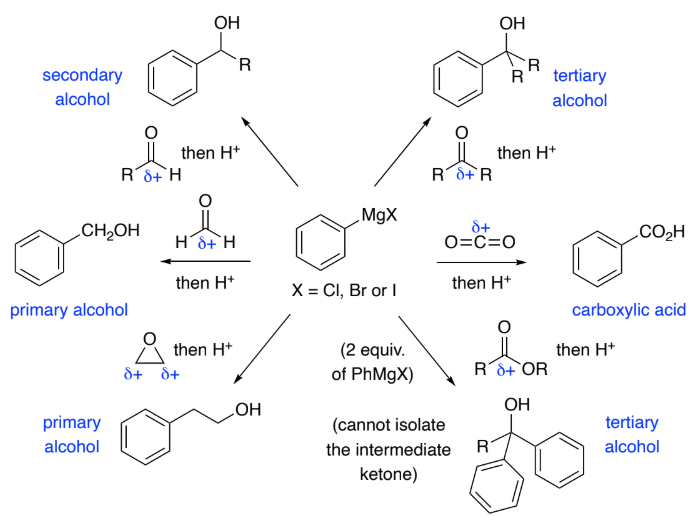
Grignards are formed by Mg insertion into an aryl halide.
They are strong nucleophiles & react with a range of electrophiles to form C–C bonds.
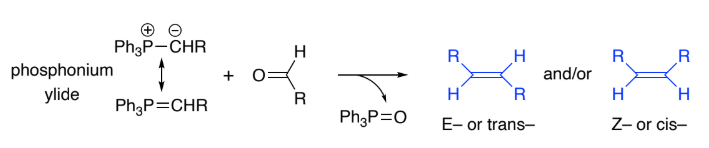
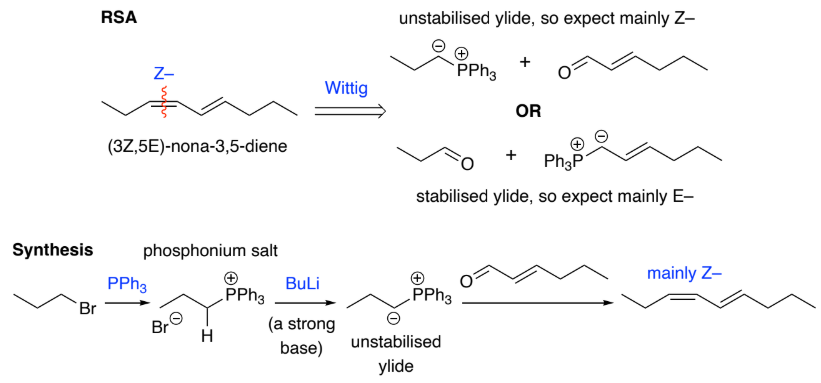
Wittig reaction is the general method for making ______ bonds.
C=C
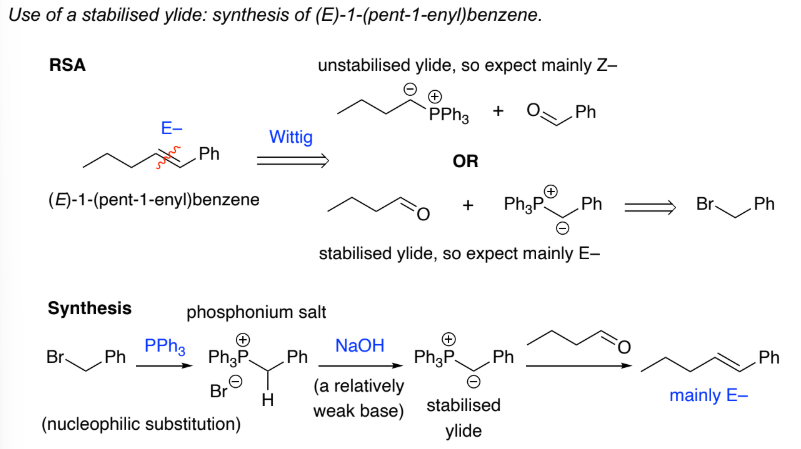
Stabilised ylides are prepared by deprotonation of a phosphonium salt, that has an EWG, Ph3P+CH2EWG w/ weak base. The EWG stabilises the carboanion formed
They:
are unreactive to ________
react with aldehydes to give mainly ___ - alkenes, why?
ketones, E
E is more thermodynamically stable
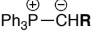
Stabilised ylides are prepared by deprotonation of a phosphonium salt, that has an EDG, Ph3P+CH2EDG w/ strong base. The EDG further destabilises the carboanion formed
They:
react with ketones & aldehydes to give mainly ___ - alkenes
Z
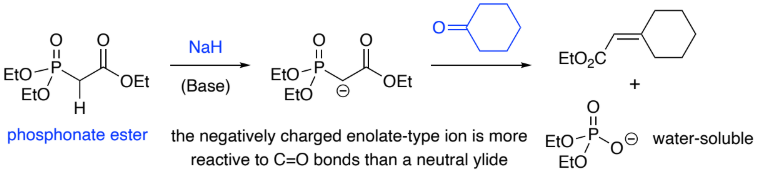
A deprotonated phosphonate ester (________ charge) is more reactive to a ____ bond than a phosphonium ylide (neutral).
Reactions of stabilised (EWG) phosphonate ester anions w/ aldehydes/ketones
gives mainly ____ - alkenes.
negative, C=O, E