MWF 320: CC1
1/293
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
294 Terms
Anti-D
Antibodies to the Rh D antigen found in serum; these antigens may signal active alloimmunization or passive immunization with Rho(D) immune globulin.
What is adminstration time frame and amount of Rh immune in pregnancy and postpartum
administration of 300 mg of Rho(D) immune globulin at 28 weeks is rec-
ommended when the fetal blood type is unknown or known to be
Rh D-positive.
what is Kleihauer-Betke
a blood test used to measure the amount of fetal hemoglobin (HbF) in the maternal bloodstream
What is adminstration time frame and amount of Rh immune in postpartum
120 or 300 mg within 72 hours of delivery
What are the testing methods for Rh neg individuals
routine blood typing, antibody screening
What is parvovirus B19
parvovirus B19 is a single-stranded DNA virus that is responsible for erythema infectiosum, a common childhood illness.
What type of infection is Parvovirus B-19
commonly spread by respiratory secretions or from hand to mouth contact. Other modes of transmission include blood product infusion and transplacental transfer
What is the Viremia (în blood stream) for Parvovirus B-19
occurs 4 to 14 days after exposure and may last up to 20 days.
What are the symptoms of Parvovirus B-19 if pregnant person is symptomatic
Erythema infectiousum rash (mostly in children)
Arthopathy
Anemia
myocarditis
flu-like symptoms, fever, and headache,
What are the symptoms of Parvovirus B-19 of fetus
Fetal loss
Anemia
Hydrops
Myocarditis
What perecntage of pregnant mother are at risk of this infection
6 The population-attributable risk of infection in susceptible pregnant women is about 55% from their own children and 6% for occupational exposure.
What perecntage of women devlop immunity to parvovirus B-19
50% to 75% of women of reproductive age have developed immunity to parvovirus B19.
What is the recommendation for mangement of Parvovirus B-19
testing for both parvovirus B19- specific IgG and IgM.
What is the difference between IGG and IGM
IGG= Good this is indicate past infection
IGM=Mal indicate acute/recent infection
What do the different results mean for when it staes neagtive or positive for IgG or IgM
Positive IgG and negative IgM indicate past infection
Positive IgG and IgM indicate infection within the last 7-120 days
Negative IgG and positive IgM indicate acute/recent infection
Negative IgG and IgM indicate that the patient is not immune and that no evidence of acute/recent infection is identified
When does the IgG vs IgM appear in testing
B19 IgM usually appears within 2 to 3 days of acute infection (10 to 12 days after inoculation) and may persist up to 6 months. Parvovirus B19 IgG appears a few days after IgM appears and usually remains present for life.9
Management of pregnant person exposed to parvovirus B19 infection
check fetal movement
When should IgG and IgM be retested
IgG and IgM tests be repeated 2 to 4 weeks later
What recomendations do we make to pregnant women with parvovirus B-19
Wash hands, masking and no kising or exchanging saliva with child, check on FM
When shpould we preform the u/s for Barovirus and what should it include
maternal–fetal medicine specialists perform ultrasonographic assessment weekly or every 2 weeks. Ultrasound assessment of the fetus should include Doppler measurement of the MCA peak systolic velocity to assess for fetal anemia.
What are some sign in u/s that fetus has been affected with Parovirus B-19
ultrasound signs of parvovirus B19 infection include increased placenta thickness, echogenic bowel/ meconium peritonitis, first trimester increased nuchal translucency, and amniotic fluid abnormalities
What is the definition of low lying placenta
Classify placental location as placenta previa (placenta covering the cervical os), low-lying placenta (edge located ≤20 mm from cervical os), or normally located placenta (edge located >20 mm from cervical os) (strong/moderate).
When should placenta previa we diagnosed
Diagnosis of placenta previa or low-lying placenta should not be made <18 to 20 weeks gestation, and the provisional diagnosis must be confirmed after >32 weeks gestation, or earlier if the clinical situation warrants.
Placenta previa
placenta touchingor covering internal os at term
Complcation of placenta previa for pregnant person
hemorrhage, hysterectomy, transfusiom,septicemia,thrombophlebitis
Complcation of placenta previa for fetal/placenta
IUGR, preterm labour, respiratory distress syndrome,low Apgar/NICU admission, anemia
Placental abruption
premature separation of placenta from wall of uterus
- > partialor complete
- > bleeding may be apparentor concealed behind placenta
Mild: evidence of abruption,
no fetal compromise
moderate: evidence of abruption,
Severe: evidence of abruption,
fetal compromise
fetal death
What are the differences of placenta previa and abruption

Vasa previa
fetal blood vessels in membrane pass across or near cervical os (in front of presenting part)
· risk of tearing during ROM or cervical dilation
· 2 types
- > in conjunction with relamentous cord insertion
- > in conjunction with bilobed or succenturiate- lobed placenta
Vasa previa risk factor
multiples
placenta previdor low-lying placenta in 2nd trimester
IVF/ART
Differntial diagnosis of bleeding
PLACENTAL ABRUPTION
VASA PREVIA
Placenta previa/marginal placenta previa
Trauma
Cervical change (rhythmic pain, not as much bleeding)
Placenta previa
What is the difference btw DAT vs IAT
Direct Antiglobulin Test (DAT), it detects whether antibodies are already attached to red blood cells.
IAT (indirect Coombs) – to see if anti-D antibodies have already formed
What is the assesmnet you perform with
ICD WTP; FM, abdominal pain, bleeding resume
choice birthplace, delivery options
BPP
Discussion on placenta 2.2cm
Labs: CBC/ferritin/platelets (hemoglobin or hematocrit are they stable?, how much blood was loss)
Transvaginal u/s
Vital
WHat test do rh neg people need
IAT (indirect Coombs) – to see if anti-D antibodies have already formed
Kleihauer-Betke test – to quantify fetal blood in maternal circulation and guide RhIG dosing
Direct Antiglobulin Test (DAT), it detects whether antibodies are already attached to red blood cells.
Why is the term marginalized placenta no longer used
it used to be detected on digital exam. And so you would feel that that margin of that placenta. And so that term is no longer being used, because now we're not detecting it on internal exam
What is Gestational Diabetes Mellitus (GDM)?
GDM is a form of glucose intolerance that develops during pregnancy and typically resolves after birth. It occurs when the body cannot produce enough insulin to meet the increased needs of pregnancy
what causes the increased insulin resistance in pregnancy?
Insulin resistance increases due to placental hormones (e.g., human placental lactogen), maternal fat deposits, and increased nutrient needs of the fetus. These changes are most pronounced in the second and third trimesters
What are the major risk factors for developing GDM?
Age ≥ 35 years
Obesity (BMI ≥ 30)
Family history of type 2 diabetes
Previous GDM or macrosomic infant (≥ 4,000 g)
Polycystic ovary syndrome
Belonging to high-risk ethnic groups (e.g., Indigenous, South Asian, Hispanic)
How is GDM diagnosed?
Screening is typically done between 24–28 weeks gestation using a two-step approach:
50 g oral glucose challenge test (non-fasting)
If abnormal, followed by a 75 g oral glucose tolerance test (fasting) to confirm diagnosis
What are the potential maternal complications of GDM?
Increased risk of hypertensive disorders (e.g., preeclampsia)
Higher rates of cesarean section
Increased risk of developing type 2 diabetes postpartum
What are the fetal and neonatal risks of GDM?
Macrosomia (birth weight ≥ 4,000 g)
Birth trauma (e.g., shoulder dystocia)
Neonatal hypoglycemia
Increased risk of childhood obesity and type 2 diabetes
How is GDM managed during pregnancy?
Nutritional counseling and dietary modifications
Regular physical activity
Self-monitoring of blood glucose
Insulin therapy if glycemic targets are not met with lifestyle measures
What postpartum care is recommended for individuals with GDM?
Blood glucose testing at 6 weeks postpartum
Ongoing screening for type 2 diabetes every 1–3 years
Lifestyle counseling to reduce long-term diabetes risk
GESTATIONAL DIABETES SCREENING AND DIAGNOSIS ALGORITHM
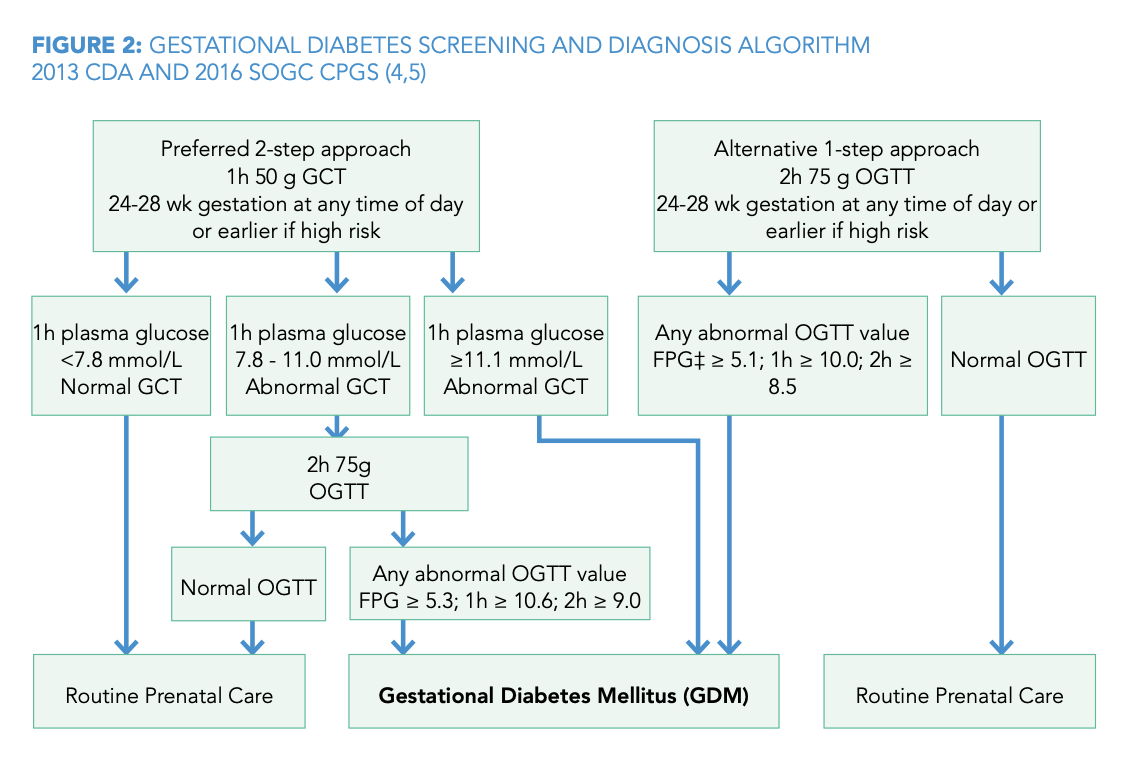
GESTATIONAL DIABETES SCREENING AND DIAGNOSIS ALGORITHM
What is the initial screening test for GDM?
50g oral glucose challenge test (non-fasting).
What is a positive result for the 50g glucose challenge test?
a plasma glucose level ≥7.8 mmol/L at 1 hour.
What glucose values confirm a diagnosis of GDM on a 75g OGTT?
fasting ≥5.3 mmol/L, 1-hour ≥10.6 mmol/L, or 2-hour ≥9.0 mmol/L.
: What is the target fasting blood glucose in GDM?
<5.3 mmol/L.
what is the target 2-hour postprandial blood glucose in GDM?
<6.7 mmol/L.
Why is glycemic control important in GDM?
To reduce the risk of fetal macrosomia and birth complications.
What fetal complication is associated with GDM?
Macrosomia.
What neonatal complication is associated with GDM?
Neonatal hypoglycemia.
What long-term risk do mothers with GDM face?
Increased risk of developing type 2 diabetes.
: How often should glucose be monitored in women with GDM?
Daily—fasting and postprandial levels.
Can GDM be managed with oral hypoglycemics?
Yes, metformin is commonly used.
Is breastfeeding encouraged in women with GDM?
Yes, it improves maternal glucose metabolism.
What is the mechanism behind insulin resistance in pregnancy?
Placental hormones like human placental lactogen antagonize insulin.
What does HbA1c indicate in pregnancy?
Long-term glycemic control but less useful for GDM diagnosis.
How is rubella transmitted?
Respiratory droplets.
What are the symptoms of rubella?
Fever, rash, lymphadenopathy, and arthralgia.
What is the incubation period for rubella?
14–21 days.
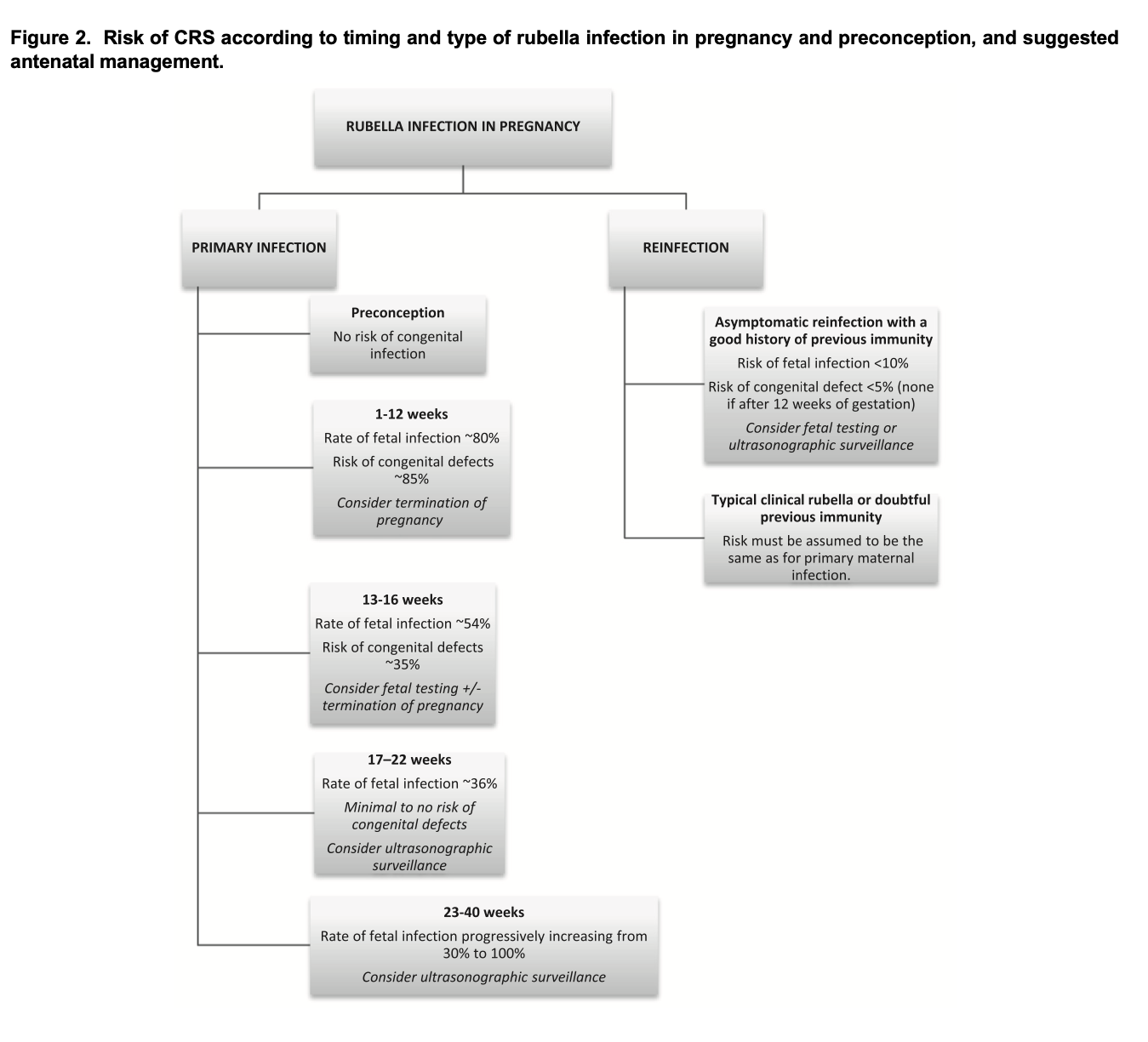
What is the major concern with rubella in pregnancy?
Congenital rubella syndrome (CRS).
During which trimester is rubella infection most dangerous?
First trimester.
What percentage risk of CRS exists if infected in the first 12 weeks?
Up to 90%.
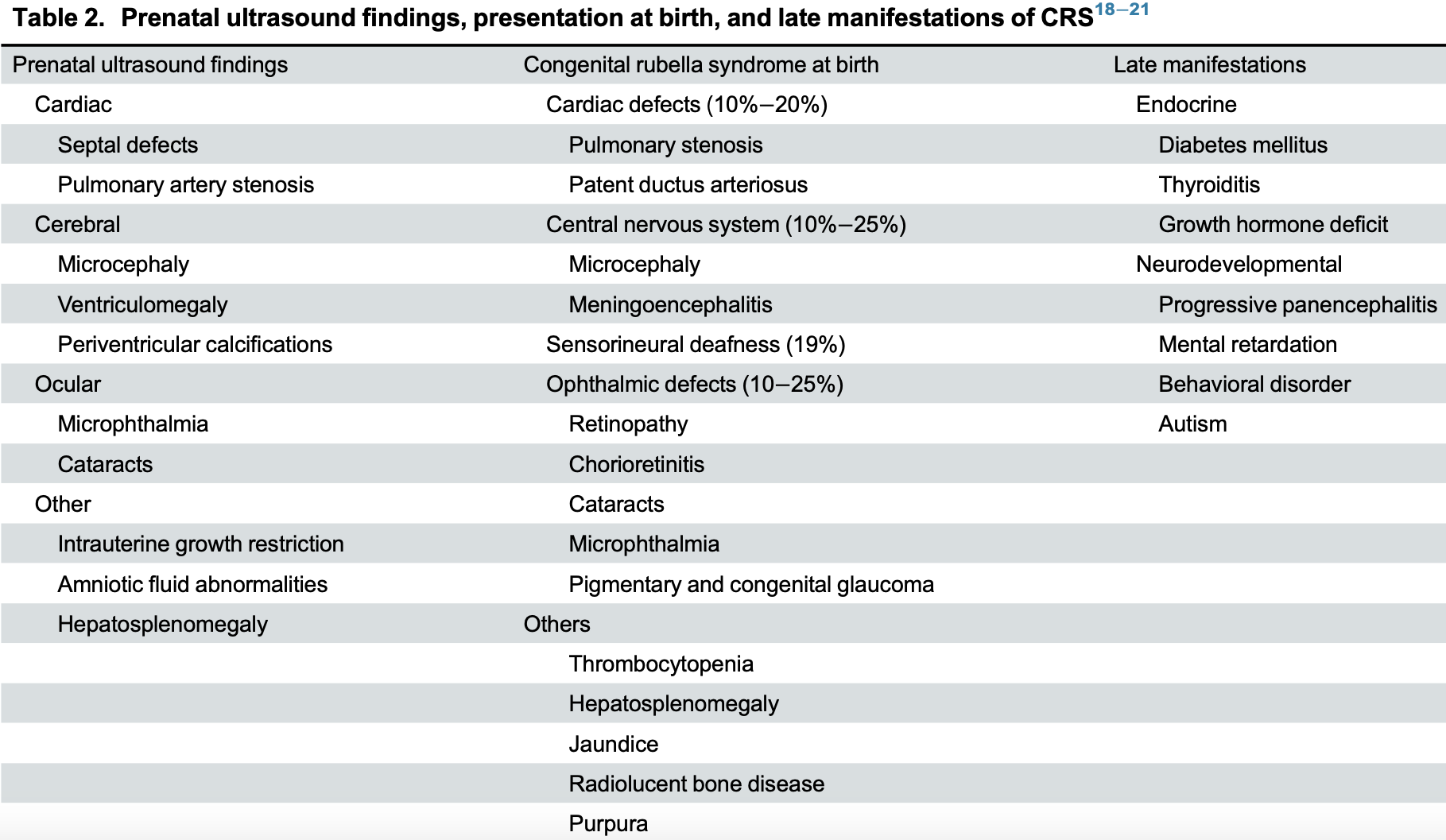
Name three features of congenital rubella syndrome.
Cataracts, deafness, congenital heart disease.
How is rubella diagnosed in pregnancy?
Rubella-specific IgM and IgG serology.
What is the management if rubella is confirmed in early pregnancy?
Offer counseling and consider termination if infection is before 12 weeks.
Can the rubella vaccine be given in pregnancy?
No, it is a live attenuated vaccine.
When is rubella immunity assessed in pregnancy?
At the first prenatal visit.
How long should pregnancy be avoided after rubella vaccination?
At least one month.
What congenital anaomlies CRS?
Patent ductus arteriosus (PDA),Congenital cataracts,Sensorineural deafness.
What is the risk of CRS if infection occurs after 20 weeks?
What are the consequences of inadequate insulin compensation in GDM?
High maternal blood glucose, risk of preeclampsia, kidney injury, fetal malformations, miscarriage, stillbirth, and macrosomia.
What is Gestational Diabetes Mellitus (GDM)?
A condition of glucose intolerance that occurs during pregnancy due to increased insulin resistance, affecting up to 14% of pregnancies.
What populations have a higher risk for GDM?
Indigenous peoples of North America, East Asian, Hispanic, and African ethnicities.
Are there clinical symptoms of GDM during pregnancy
Typically no; possible findings include excessive gestational weight gain and large for dates fundal height.
What are target blood glucose values in GDM management?
Fasting: ≤5.3 mmol/L
1-hour postprandial: ≤7.8 mmol/L
2-hour postprandial: ≤6.7 mmol/L
What are the recommended dietary targets for GDM clients?
30 kcal/kg/day (22 for obese clients), with 20% protein, 40% fat, and ≤40% carbohydrates; focus on low glycemic index foods.
What is the significance of HgbA1c in pregnancy?
It reflects average blood sugar over 2–3 months. Higher levels correlate with increased risk of fetal malformations.
What are the risks associated with high HgbA1c levels (>6%, >14%)?
>6%: 2.7% risk of malformations
14%: up to 20% risk
Increased cardiac, musculoskeletal, and nervous system defects.
Shoulder dystocia
Shoulder dystocia is when one or both shoulders are trapped above the pelvic brim after the head has
delivered. Almost always it is only one shoulder, the anterior shoulder is impacted above the symphysis
pubis. Posterior shoulder dystocia can occur from impaction of the posterior fetal shoulder on the sacral
promontory.
incidence of shoulder dystocia
suggests that shoulder dystocia occurs in approximately 0.6% to 2.1% of all
spontaneous births, and that this rate has risen steadily over time.
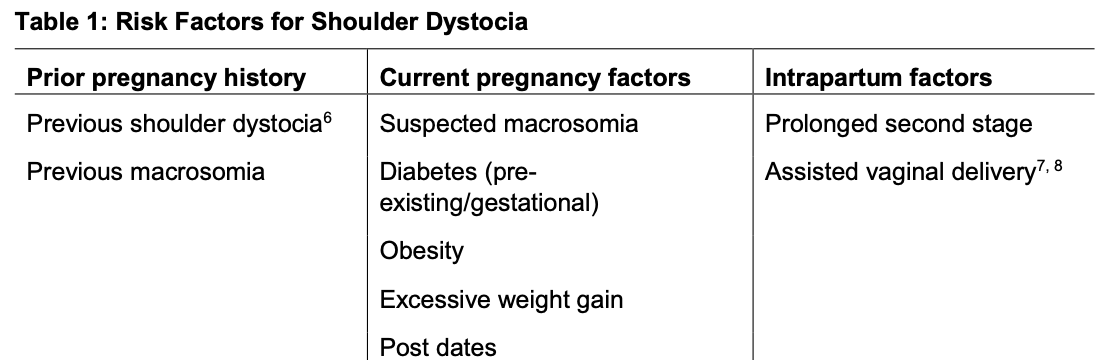
Risk factor of shoulder dystocia
Fetal/Neonatal of shoulder dystocia
Complications of shoulder dystocia include neonatal brachial plexus palsy (NBPP) (10-20% of shoulder dystocia cases, there is a brachial plexus injury.), fractures of the
clavicle and humerus, hypoxic ischemic encephalopathy, and rarely fetal/neonatal death.
Maternal complication of shoulder dystocia
Perineal tears may be severe and can occur with appropriate management secondary to the
manoeuvres required to free the impacted shoulder. There is an increased risk of obstetrical anal
sphincter injury (OASI).17, 18 Uterine atony and postpartum haemorrhage are common occurring in 10 –
20 percent of cases. Maternal symphyseal separation and lateral femoral cutaneous neuropathy have
been reported likely secondary to hyperflexion of the hips.
Diagnosis of shoulder dystocia
Shoulder dystocia can be suspected if the fetal chin delivers with difficulty and the head recoils against
the perineum (‘turtle sign’). Spontaneous restitution does not occur. The mother fails to deliver the
anterior shoulder with her own expulsive efforts during the next contraction.
estimate that rates of shoulder dystocia during a cephalic spontaneous birth are:
< 4000 g: 1%
4000 to 4500 g: 5%
4500 to 5000 g: 10%
> 5000 g: 20%
Associated factors for shoulder dystocia
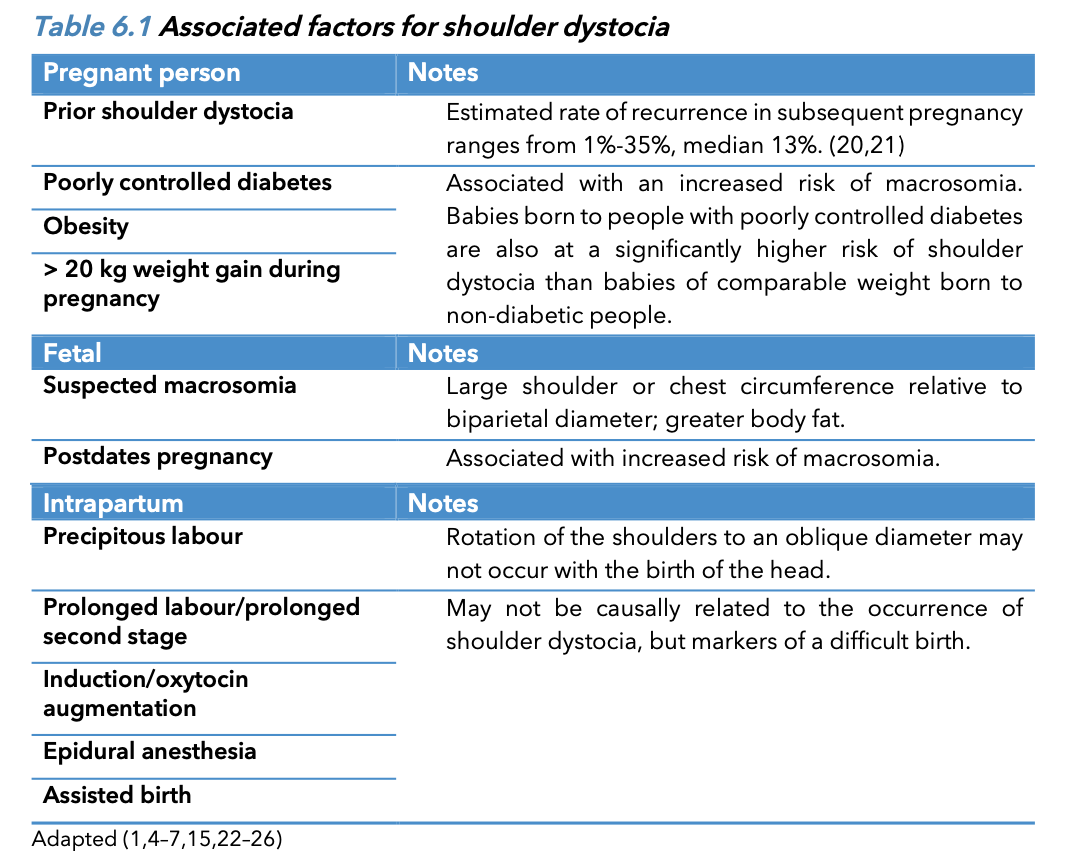
Associated c omplications for the p regnant p erson
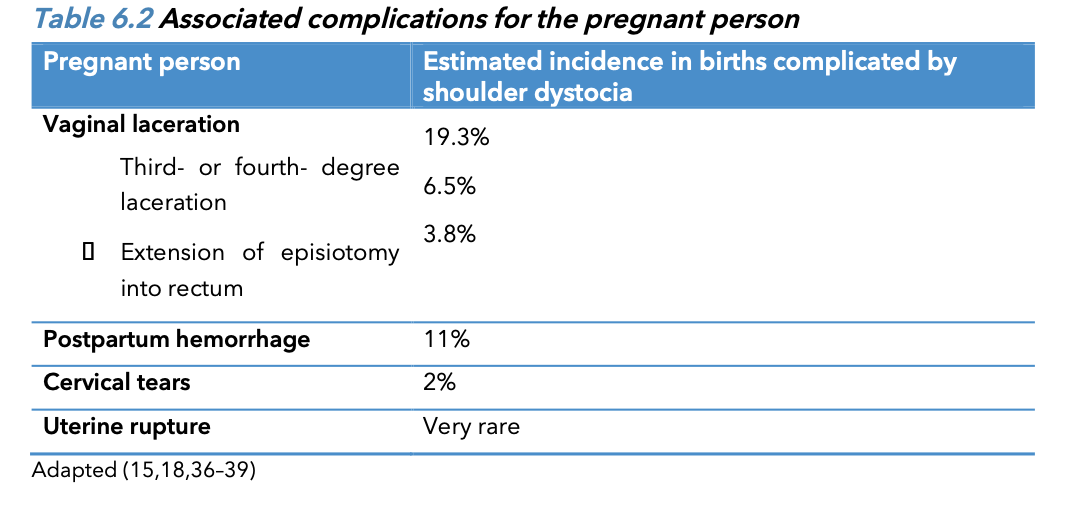
Associated c omplications for baby
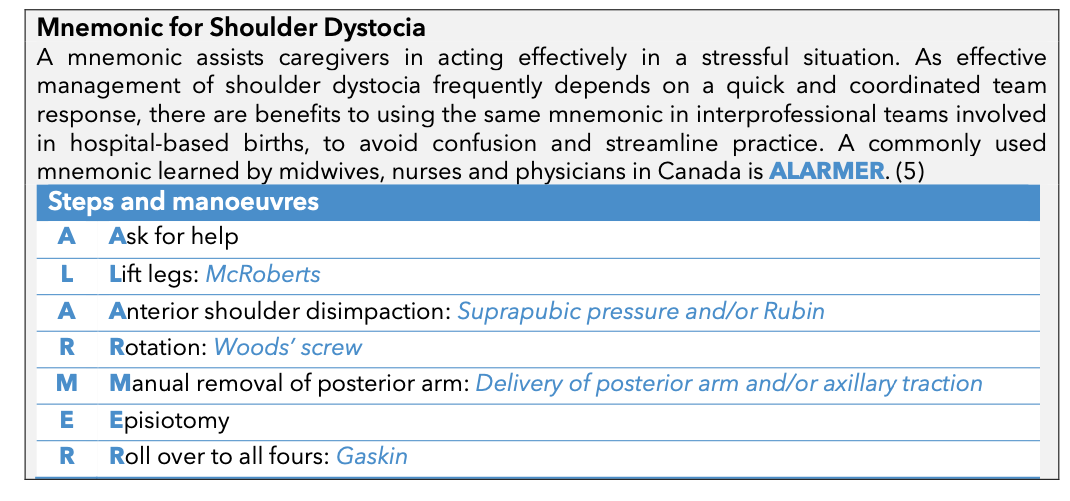
Mnemonic for Shoulder Dystocia
Why is neonatal hypoglycemia difficult to define with a single glucose value?
Because glucose levels vary based on gestational age, size, clinical condition, and energy needs, and because signs may overlap with other conditions.
What is Whipple’s triad and why is it impractical in neonates?
It includes symptoms of hypoglycemia, low serum glucose, and symptom resolution with glucose. It's hard to apply in neonates due to vague symptoms and difficulty confirming symptom resolution.
What are common signs of neonatal hypoglycemia?
Jitteriness, cyanosis, seizures, apnea, tachypnea, high-pitched cry, limpness, poor feeding, eye-rolling, pallor, sweating, hypothermia, cardiac failure.
Name at least five risk factors for neonatal hypoglycemia.
SGA (<10th percentile)
LGA (>90th percentile)
Preterm <37 weeks
Infants of diabetic mothers (IDMs)
Maternal labetalol use
What blood glucose value is commonly used to define hypoglycemia in healthy infants in the first 72 hours?
<2.6 mmol/L
What is the therapeutic glucose goal for persistent hypoglycemia after 72 hours?
≥3.3 mmol/L
How do blood glucose levels typically change in healthy, breastfed term infants after birth?
Drop to as low as 1.8 mmol/L at 1 hour, rise above 2.0 mmol/L and stabilize by 72 hours.
Why must point-of-care glucose testing in neonates be interpreted cautiously?
These methods can be inaccurate at low glucose levels and affected by sample errors or delays.