OAT Booster Gen Chem Formulas
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Solutions:
Molarity
Molality
Normality
Mole Fraction of Solute
Boiling Point Elevation
Raoult’s Law
Freezing Point Depression
Osmotic Pressure

Gases:
Ideal Gas Law
Graham’s Law of Effusion
Dalton’s Partial Pressure
Gas Density
Combined Gas Law
Avogadro’s Law
Boyle’s Law
Charles’ Law
Gay-Lussac’s Law
Dalton’s Law
BL: PV = PV
CL: V/T = V=T
GLL: P/T = P/T

Thermochemistry & Thermodynamics:
Heat Equation
Change in Energy Equation
Work Formula
Bond Dissociation Energy
Enthalpy of Formation
Entropy

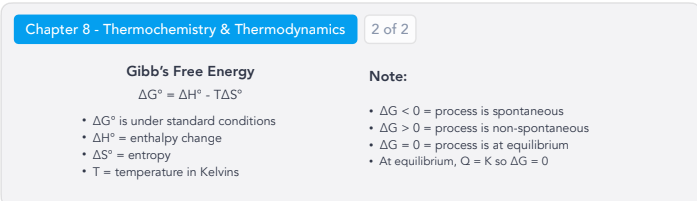
Thermochemistry & Thermodynamics:
Gibb’s Free Energy
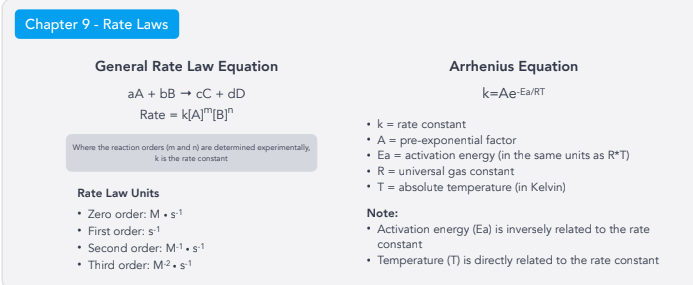
Rate Laws:
General Rate Law Equation
Rate Law Units
Arrhenius Equation
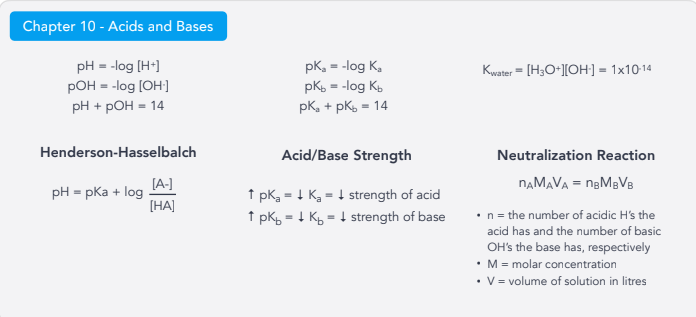
Acids/Bases:
pH/pOH
pKa/pKb
Kwater
Henderson-Hasselbalch Equation
Neutralization Reaction
CARDIO
Charge
Atom
Resonance
Dipole Induction
Orbitals
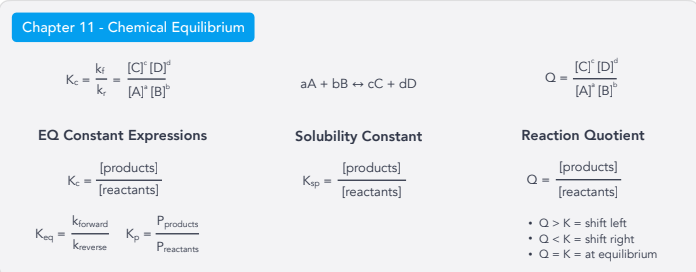
Chemical Equilibrium:
K and Q
EQ Constant Expressions
Ksp Solubility Constant
Reaction Quotient
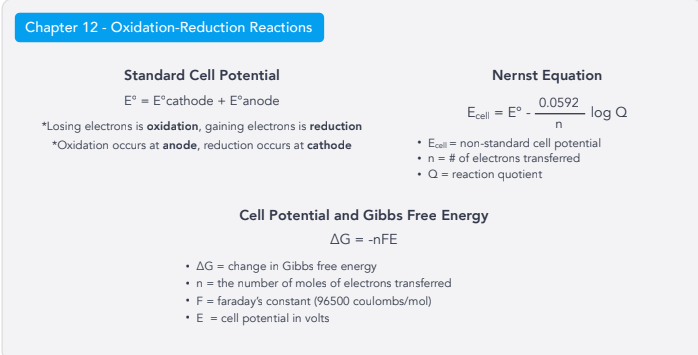
Oxidation-Reduction Rxns:
Standard Cell Potential
Nernst Equation
Cell Potential & Gibbs Free Energy
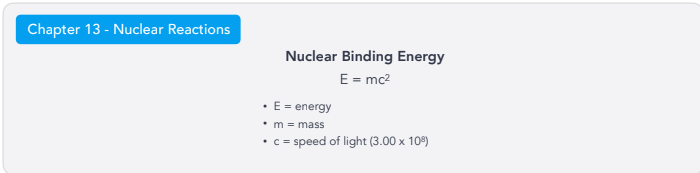
Nuclear Binding Energy
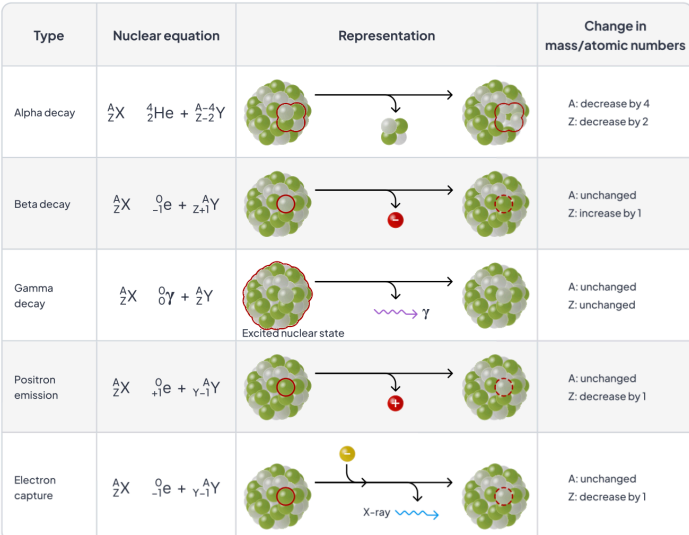
alpha decay
beta decay
gamma decay
positron emission
electron capture
Avogadro’s Number
Faraday’s Constant
moles of gas to liters at STP
atm to mmHg to torr
