Creams, ointments and gels
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
What are creams?
Semi-solid preparations intended for external use
What characteristics do creams, ointments, gels and pastes usually have?
Non-Newtonian characteristics e.g., plastic, pseudoplastic or thixotropic type flow
What are aqueous creams?
O/w emulsions
What are oily creams?
W/o emulsions
What is the aqueous phase of a cream structured by?
adding structuring materials e.g., clay particles, polymers
Forming lamellar gel network phases due to emulsifier and water reactions
What general properties do creams have?
Miscible with skin secretion, for protective and therapeutic purposes
Why are some bases called creams?
Some water-miscible bases that have a complex matrix like structure have a similar cream appearance and consistency but aren’t scientifically creams
What are hydrophilic creams?
Aqueous phase as the continuous phase - O/W emulsions that aren’t greasy, water washable, non-occlusive and the continuous phase evaporates leaving the residue of drug on the skin
Why are hydrophilic creams non-greasy and water washable?
External phase is water - feeling the cream feels more like water and water can wash it away
What are Lipophilic creams?
Oil as continuous phase, W/O emulsions, greasy texture, not water washable, occlusive
What are the most commonly used emulsifiers for creams?
lipophilic amphiphiles e.g., fatty alcohols or fatty acids in large quantities
Water soluble surfactants - ionic or non-ionic
What must you keep in mind when choosing emulsifiers?
Keep HLB in mind and check surfactants chemical structure
What are some examples of emulsifiers for creams?
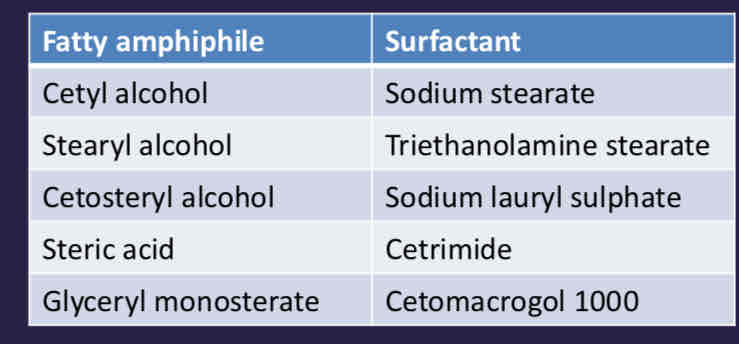
What are some examples of ready made mixtures for creams?

What are stability of creams affected by?
droplet size
Creaming
Coalescence
What can affect cream stability at higher temperatures?
interfacial energy/tension
Interfacial film of emulsifiers
Charge repulsion
Steric stabilisation
What does the external phase form in gel network structure?
Forms a certain structure where droplets will be immobilised which stabilises the creams
What can the structure of the external phase be due to?
gel forming polymers
Lamellar gel network theory
Excess amount of lipid amphiphiles used in creams so more than can be adsorbed on o/w interface
Alpha crystalline gel-network phase formed upon contact with water
Self-bodying effect - more fluid at low concentrations, more rigid at higher concentrations
Interaction between emulsifiers in water is key
What are the 3 polymorphs of long chain alcohols?
Alpha, beta and gamma
What is the alpha form of alcohols?
Come out after being at high temperature and come out first when cooled - only stable over a narrow temperature range
What are beta and gamma forms of alcohol?
Lower temperatures - 2 forms can coexist
What is reduced when lipid amphiphiles are in mixture form?
Transition temperature is reduced
What does cetyl/stearyl alcohol exist as at room temperatures?
Beta and gamma crystalline polymorphs
What does cetostearyl alcohol exist as at transition temperatures?
Alpha crystalline forms
What does the formation of alpha crystalline lead to?
Forming liquid crystalline and swollen crystalline phases
What is the lamellar gel network theory?
How the bilayer forms between water and its surfactants
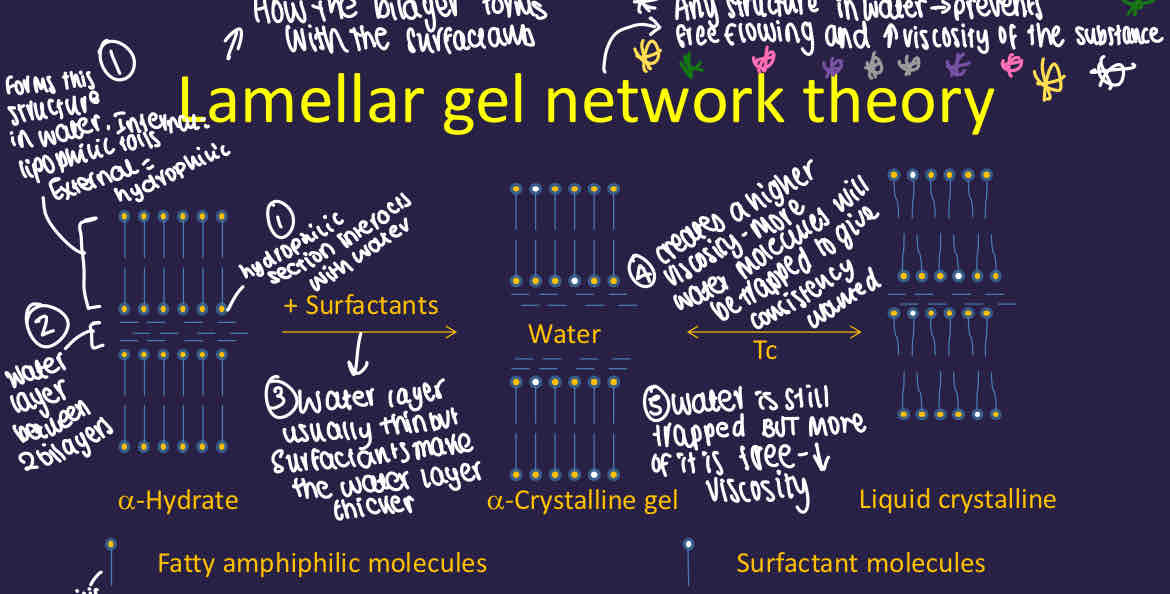
How does the lamellar gel network theory occur?
Water forms a structure where the hydrophobic heads are inside and the hydrophilic section interacts with water
There is a water layer between 2 bilayers which is usually thin
Surfactants are added which makes the water layer thicker, increasing viscosity as more water molecules will be trapped and is a viscoelastic gel phase
After Tc - water is still trapped but more is free and viscosity decreases, forming a less swollen liquid crystalline state
The crystalline gel network traps and immobilises oil droplets and stabilises the cream
What happens if you have any structure in water?
Prevents free flowing and increases the substances viscosity
What does a-crystalline form in the presence of water?
Waxy crystalline hydrates with limited swelling
What does a-crystalline form in the presence of small amounts of surfactants (10-30:1)?
Forms a-crystalline gel phase
What form does an a-crystalline gel phase change to when heating to Tc?
A less swollen liquid crystalline form
What is Tc?
Gel liquid transition temperature
What happens when creams are cooled below Tc?
Reverts back to the swollen gel phase
What is Tc usually between?
40-50 degrees Celsius for cetostearyl alcohol and other commonly used amphiphiles in creams
What is the cream structure like in higher temperatures when manufactured in terms of viscosity compared to when it has cooled after manufacture?
Less viscous due to liquid crystalline state in manufacture but cools and becomes more viscous
What is a diagram showing the microstructure of creams?
Shows the gel structure despite bulk water - only a small amount of water trapped here and there
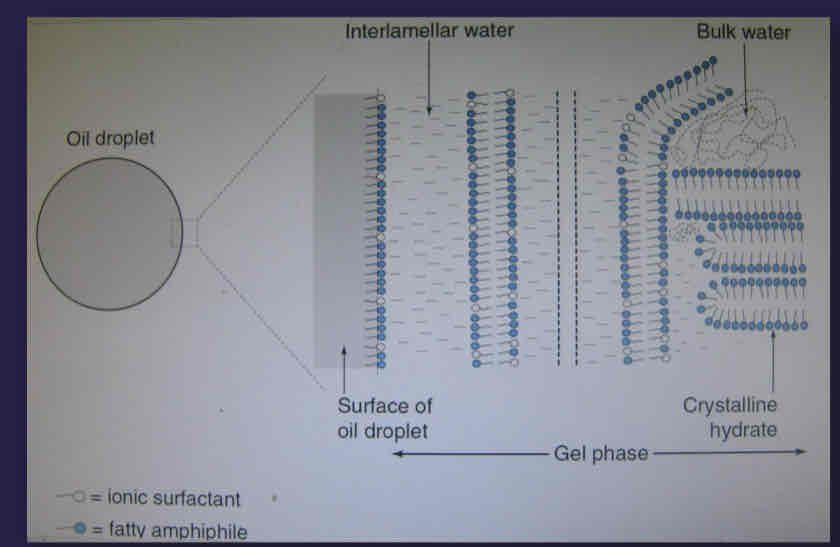
What are dispersed oil droplets stabilised by with creams microstructure?
monolayer emulsifier film
Charge
What is the microstructure of creams?
dispersed oil droplets
Viscoelastic continuous phase
What is the viscoelastic continuous phase in a-crystalline gel phase?
Bilayers of fatty alcohol and surfactant separated by interlamellar fixed water and has significant swelling
What is the viscoelastic continuous phase for a-crystalline hydrates?
Has limited swelling an has a bulk continuous free water
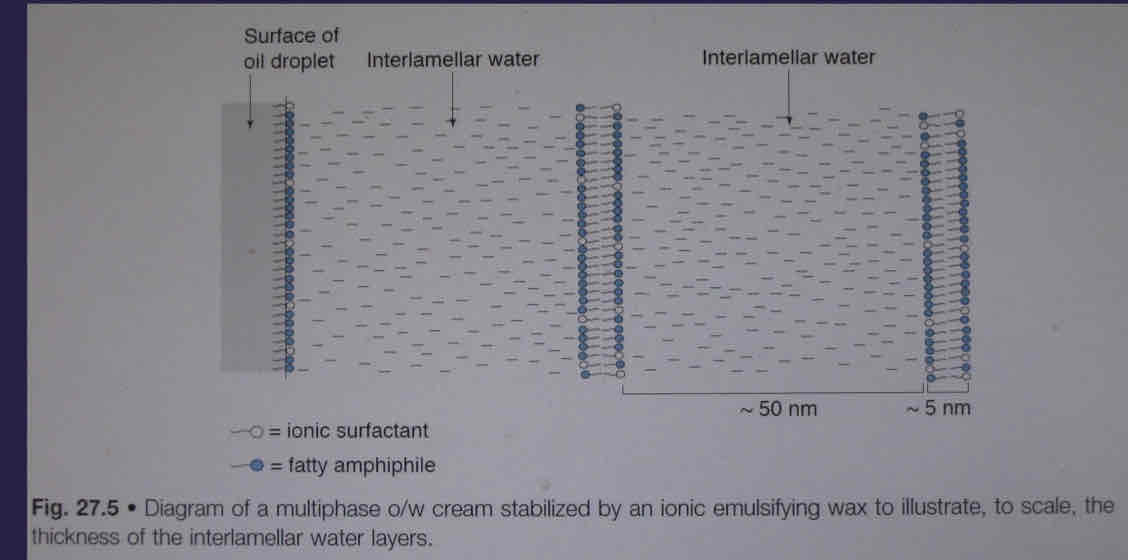
What is fatty alcohol with ionic emulsifiers?
show extensive swelling - water layer is 10x thicker than carbon layer as amphiphiles trap the water due to charge creating space from the ionic emulsifiers
Electrically charged - DLVO theory applies to adjacent bilayers
What will adding electrolytes do the fatty alcohol with ionic emulsifiers?
Compressing electric diffuse double layer reduces the adjacent bialyers which reduces volume of lamellar gel-network phase and reduces viscosity
What is a diagram showing fatty alcohol with ionic emulsifiers?
Emulsifiers trap lots of water between the bilayers which increases viscosity
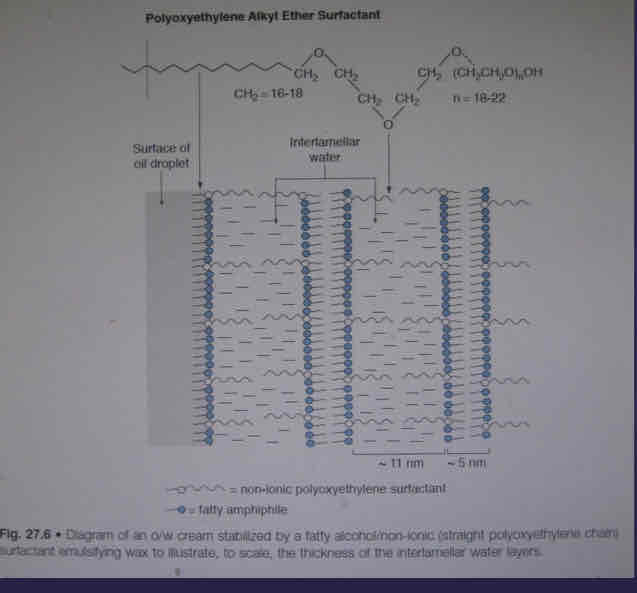
What are fatty alcohol with polyoxyethylene surfactants?
swelling due to hydration of PEO chain
Steric stabilisation from PEO chain that extends into the water layer
What does increasing temperature do to fatty acid with PEO chains?
PEO chain is less well hydrated so no gel phase is formed and is more fluidic
What does storage at lower temperatures of PEO chains do to fatty acids with PEO surfactants?
PEO is better hydrated and has more swelling due to gel phase forming and is more viscous
What is the diagram showing fatty alcohol with PEO surfactants?
What are fatty acid mixed emulsifiers?
Fatty acids have different polymorphs - can make vanishing creams that leave non-greasy residue on the skin
What are stearate creams?
Stearic acids partially neutralise by alkali - add triethanolamine bit
What is the effect of adding alkali groups to fatty acids?
Adding alkaline group makes it become ionised and creates a hydrophilic emulsifier - COOH group can become COONa when NaOH added
What are the perceivable attributes of creams?
overall consistency
Cosmetic appearance
Rheological properties
Rheological stability over a period of time - thinning or thickening
What affects the perceivable attributes of creams?
Structure
What structure factors can affect the attributes of creams?
mechanisms and kinetics involved in formation of various phases
Thickness of interlamellar water layers
Proportion of added water that is between lamellae
Relative proportions of various phases - not just water
Stability of various phases over different temperatures and batch variation of components
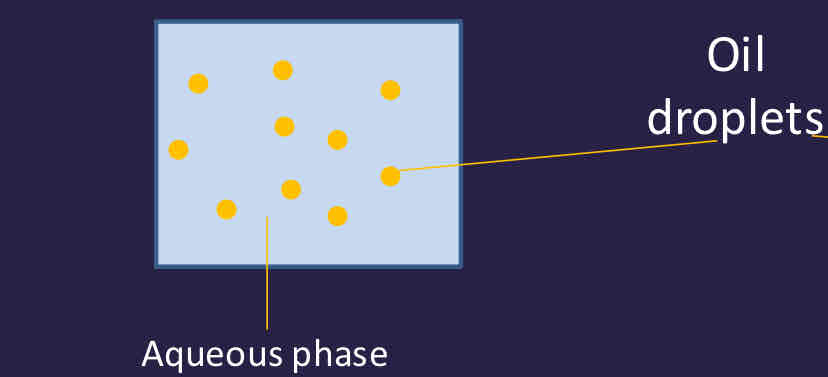
What is a lipid emulsion?
Oil droplets free move - can coalesce, can flocculate and can move to top forming a cream layer
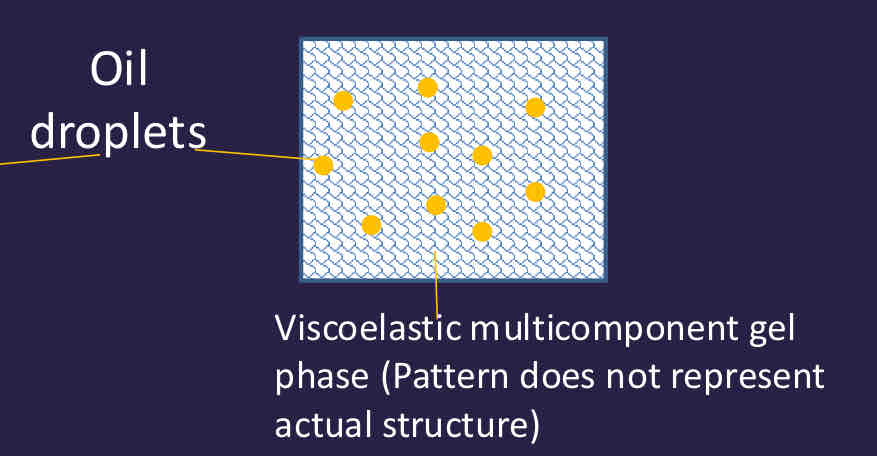
What are creams in comparison to lipid emulsions?
Oil droplets immobilised in gel phase so has no creaming, coalescence or flocculation - gel phase structure is important
What are the rheological properties of creams dependent on?
Concentration of mixture of emulsifiers due to swelling properties of lamellar gel network phase
What is self-bodying action at low emulsifier concentration?
Structured liquid and high proportion of free water so is more fluidic
What is self bodying action at high emulsifier concentrations?
Increased proportion of swollen lamellar gel-network phase, reduced free water proportion and is more viscous
Why are creams manufacture more complex than liquid emulsions?
Due to formation of structures in continuous phase which are sensitive to dynamic manufacture process
What must be considered when manufacturing creams?
scaling up must be carefully controlled
Each type of equipment introduces energy into system in a different way
Creams prepared from large production equipment could be very different from smaller lab equipment
Other variations e.g., heating and cooling cycles
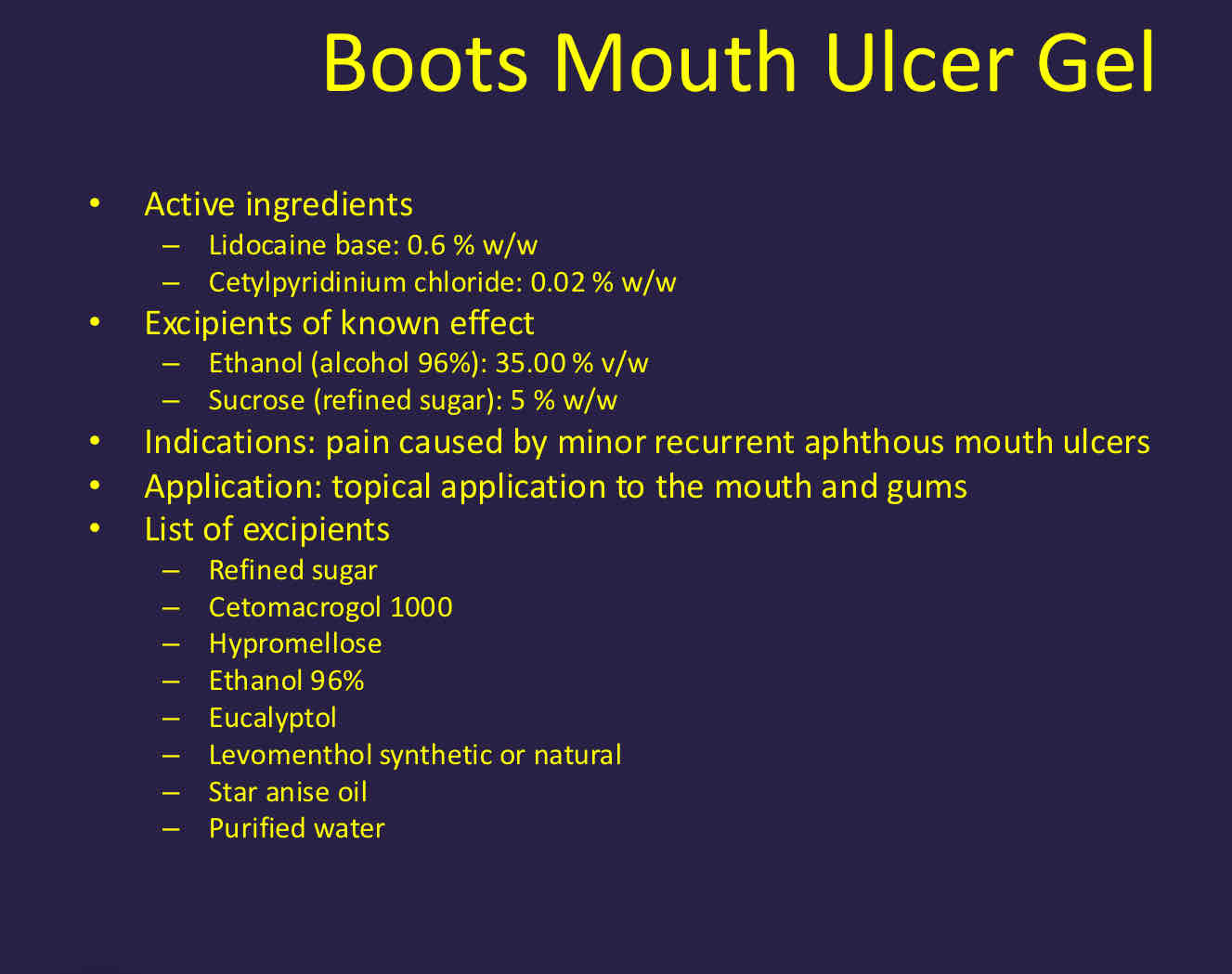
What is terbinafine 1% cream?
10mg terbinafine hydrochloride and is for cutaneous use
Excipients - benzyl alcohol, cetostearyl alcohol, cetyl alcohol
Long list of excipients - cetyl palmitate, cetyl alcohol, cetostearyl alcohol as lipophilic amphiphiles, polysorbate 60, sorbitan monostearate as surfactants, Sodium hydroxide, purified water
What is Canesten cream?
Clotrimazole 1% that has various indications for moulds, fungi, yeasts, secondary nfectin and nappy rash etc and is applied thinly on hands or feet. Has a long list of excipients

What are ointments?
Consist of a single-phase base where solids or liquids may be dispersed
What are the properties of ointments?
Highly viscous and moisture occlusive - emollient, protective and therapeutic effects
What are medicaments?
Can be dissolved or dispersed in ointments
What are hydrophobic ointments?
Can absorb only a small amount of water and has an occlusive effect - typical bases include heavy liquid and light liquid paraffins, vegetable oils, animal fats, synthetic glycerides, waxes etc
What are water-emulsifying ointments?
Can absorb larger amounts of water and results in w/o or o/w emulsions with amphiphilic materials
What are hydrophilic ointments?
Bases are miscible with water e.g., polyethylene glycols and may contain certain amounts of water - less emollient
How do you prepare ointments?
Melt the base at high temperatures - above melting point
Mix excipients and drugs
Cool them and avoid separation of different materials
May need special equipment e.g., rollers to maintain smoothness
What is aciclovir agepha 30mg/g eye ointment?
30mg/g aciclovir for herpes simplex keratitis - ocular use and has 1 excipient - white soft paraffin as it is for the eyes so less excipients
What are gels?
Semi-solid systems where colloidal particles may link together to form a 3D network formation that also has water molecules held within it
How much water can hydrogels/some gels hold?
Around 90% water
What properties do gels have when applied?
Thin after application of small shear stress and are amenable to topical application. Look at shear thinning
What are type 1 gels?
covalent bonds between macromolecules
Irreversible system where the cross link created by polymers isn’t dissolvable
Polymeric implants and has sustained release of drugs
E.g., 2-hydroxyethylmethacrylate HEMA crosslinks with ethylene glycol dimethacrylate
How are gels formed?
Gelation of lyophilic colloids - polymers
What are type 2 gels?
hydrogen bonds or van der Waals forces
Heat-reversible - heating or cooling
E.g., polyvinyl alcohol
What type of gels will show a change in viscosity when a shear stress is applied?
Type 2 due to H bonds
What type of flow do hydrophilic gels tend to demonstrate?
Shear thinning
What is gelation of lyophobic colloids?
Particles of lyophobic colloids link together to form gels e.g., bentonite, aluminium magnesium silicate
What forces are present in gelation of lyophobic colloids?
Van der Waals forces and electrostatic attraction between particles
What happens to gelation of lyophobic colloids when shear stress is applied?
Shear stress being applied leads to lyophobic colloids with lower viscosity - liquid formed. Thixotropy
What are some example of semi-synthetic materials?
methylcellulose - MC
Carboxymethylcellulose - CMC
Hydroxyethylcellulose - HEC
Hydroxypropylcellulose - HPC
Hydroxypropylmethylcellulose - HPMC
What are some other gelling agents?
natural gums - acacia, tragacanth, xanthan, alginate
Clays e.g., bentonite
Synthetic materials e.g., carbomer
Less commonly used
What is tragacanth as a natural gum gelling agent?
Mixture of water-insoluble and water soluble polysaccharides which are negatively charged in aqueous solution
What happens at Tg/gelling temperature?
Some polymer solutions will turn into a gel at a given temperature upon heating or cooling - polyvinyl alcohol turns into a gel upon cooling
Is gelling temperature melting point?
NO! Different things - gelling temperature is usually a range of temperatures
What will increase gelling temperature?
Increase gelling agent concentration
What does a higher gelling temperature mean?
A higher energy is needed to break the structure of the gel network
What happens to concentrated poloaxmer solutions when heated?
Turn into gels - forms micelles at high concentrations above CMC and polymer chains better hydrated at low temperatures. Increasing the temperature reduces solubility so more micelles formed at higher temperatures which compact together to form gels
What are carbomers?
Contain 56-68% w/w Carboxylic acid groups
What happens when you add an alkali material to Carboxylic groups in carbomers?
adding NaoH or KOH increases ionisation for aqueous or polar solvents - makes it more hydrated
What can you add for carbomers if they are less polar or non-polar solvent systems?
Add triethanloamine or diethanolamine
What happens to the Carboxylic acid group when alkali’s or triethanoloamine or diethanolamine are added for carbomer uncoiling?
COOH groups are converted to COO-Na+
What does viscosity of the resultant gel depend on in carbomers?
Extent of polymer opening
What happens at a higher pH with carbomers when alkali has been added?
Carbomer uncoils and is easier for them to cross over and give a 3D network - COO- present
What happens at lower pH for carbomers?
Randomly coiled polymers, COOH
What is an example for gels?
Sugar for taste, more excipients so the patient will take it