3.13 Beer-Lambert Law
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
One way that we can measure the concentration of a colored substance is by:
Seeing how much light can be passed through it or how much light is stopped by the solution
The measure of the light that is stopped by or absorbed by a solution is referred to as the:
Absorbance
A ______ can be used to measure the absorbance.
Spectrophotometer or a colorimeter
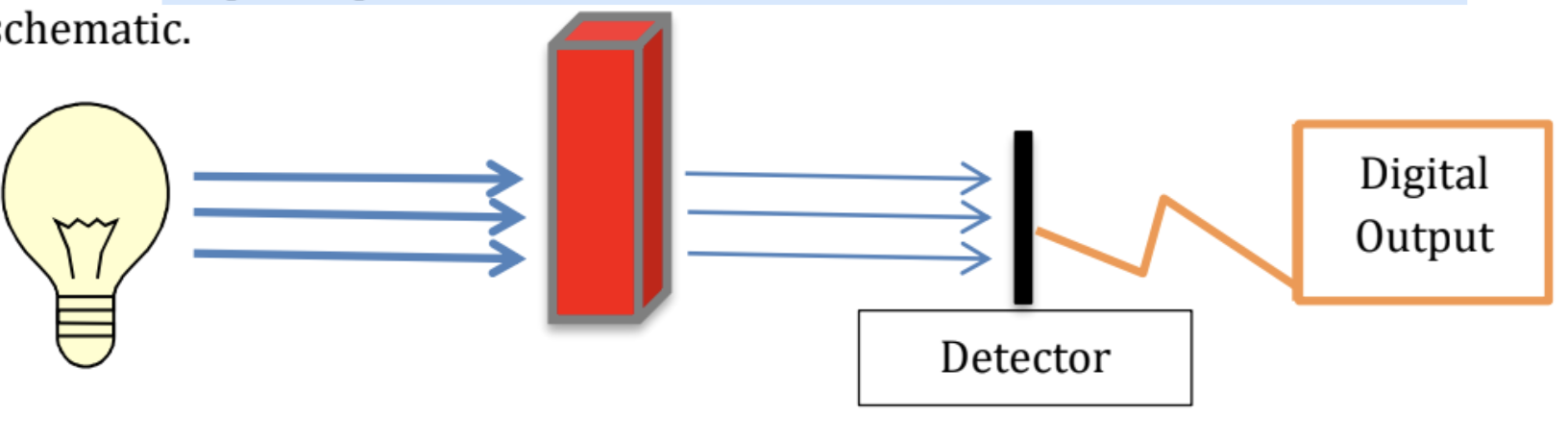
Simplified schematic of spectrophotometer or colorimeter:
The more concentrated the solution is, the more/less light can pass through the solution
Less
A measure of the amount of particles that are distributed in a solution
Concentration
You can think of concentration as:
How densely packed the particles are
Concentration is measured in a lot / a few of ways
A lot
Chemists use ______ to measure concentration
Molarity
What is molarity in terms of solute and solution?
Moles of solute divided by the volume of solution
The term used for the substance that is dissolved
Solute
Most commonly we use ______ as the solvent
Water
The solute and solvent together are called the:
Solution
A solution with water as the solvent is:
An aqueous solution
Units for molarity:
M, mol/L
Can molarity be used as a conversion factor?
Yes
Why can molarity be used as a conversion factor?
It has two units, moles and liters
Different colors of solutions absorb different:
Colors (or wavelengths) of light
How to measure a red solution?
By using green light
Wavelength of green light:
560-520 nm
Red and green are:
Complementary colors
For colored solutions, ______ can be used to measure the concentration of solutions
Absorbance
To measure absorbance, you first have to:
Determine the best wavelength to use to measure your sample
How to determine the best wavelength to use to measure your sample?
Place your sample into a spectrophotometer and generate a graph of absorbance vs. wavelength
On a graph showing absorbance vs wavelength, the best choice for a wavelength is one where the absorbance is:
Close to 1
What should you do after determining the wavelength used for measuring absorbance?
Create a calibration graph with standard solution concentrations and their corresponding absorbance value
Calibration graph with concentrations and corresponding absorbance values should be:
Linear
The slop of the calibration graph with concentrations and corresponding absorbance values gives the:
Molar absorptivity constant
The more concentrated a solution is the more/less light is absorbed by the solution
More
Beer-Lambert Law:
A = ԑ b c. (A = Absorbance, ԑ = molar absorptivity constant (1/M*cm), b = path length (usually 1 cm), c = concentration (Molarity, M))
How to determine concentration of unknown sample using absorbance?
Using the calibration graph for the solution
In Beer-Lambert Law, the path length is:
Width of the cuvette that the sample is placed in
Is path length usually held constant?
Yes
When you calibrate the spectrophotometer you should start with:
A “blank”
The “blank” on the spectrophotometer is:
Your solvent only, usually water
By calibrating the spectrophotometer with the blank it will:
Remove the data from the solvent
By removing data from the solvent, the spectrophotometer:
Only outputs the absorbance of the solute
Calibrating the spectrophotometer with the blank is analogous to:
The “tare” button on a digital scale