CSF Exam 3
4.8(5)
4.8(5)
Card Sorting
1/124
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
125 Terms
1
New cards
What are the two pathways that make up the metabolism?
catabolic and anabolic pathways
2
New cards
What is a catabolic pathway?
It is a (breaking down) pathway that breaks down food particles into smaller molecules generating energy and building blocks
* aerobic respiration
* aerobic respiration
3
New cards
What is an anabolic pathway?
it is a (biosynthetic) pathway that uses small molecules along with energy harnessed from catabolism to drive the biosynthesis of other molecules
* photosynthesis
* photosynthesis
4
New cards
What is oxidation?
refers to removal of electrons
5
New cards
What is reduction?
refers to gaining of electrons
6
New cards
What makes a reaction energetically favorable?
reactions that decrease the free energy ( - delta G)
7
New cards
What makes a reaction energetically un-favorable?
reactions that increase the free energy ( + delta G)
8
New cards
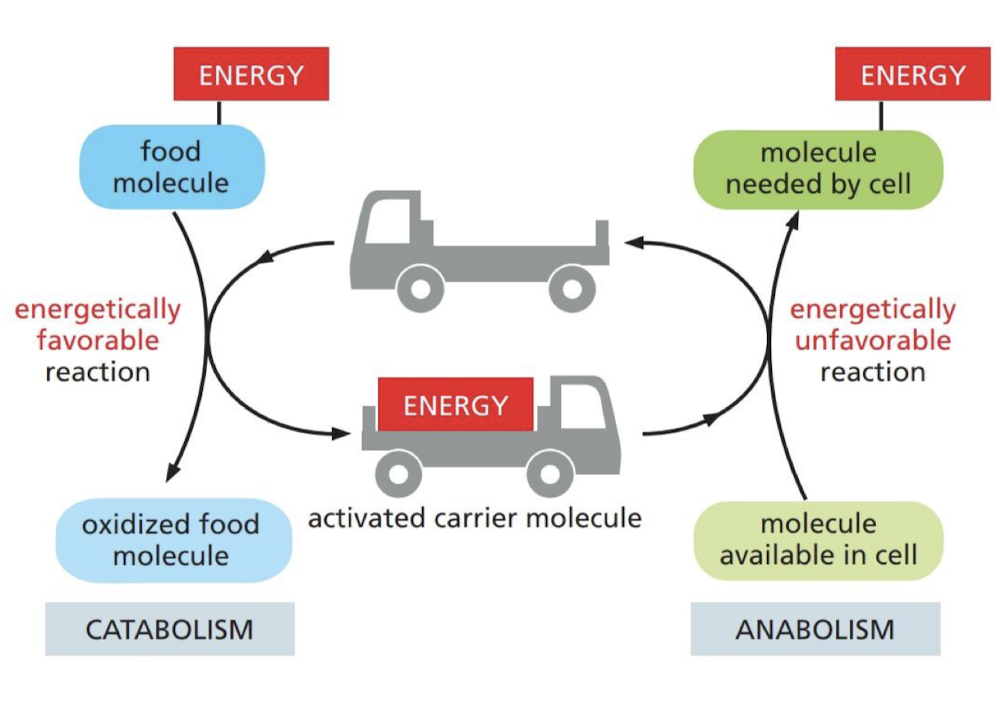
How is energy released from reactions stored in our cells?
chemical-bonding energy in carrier molecules store energy in covalent bonds of ions (chemical groups or electrons)
9
New cards
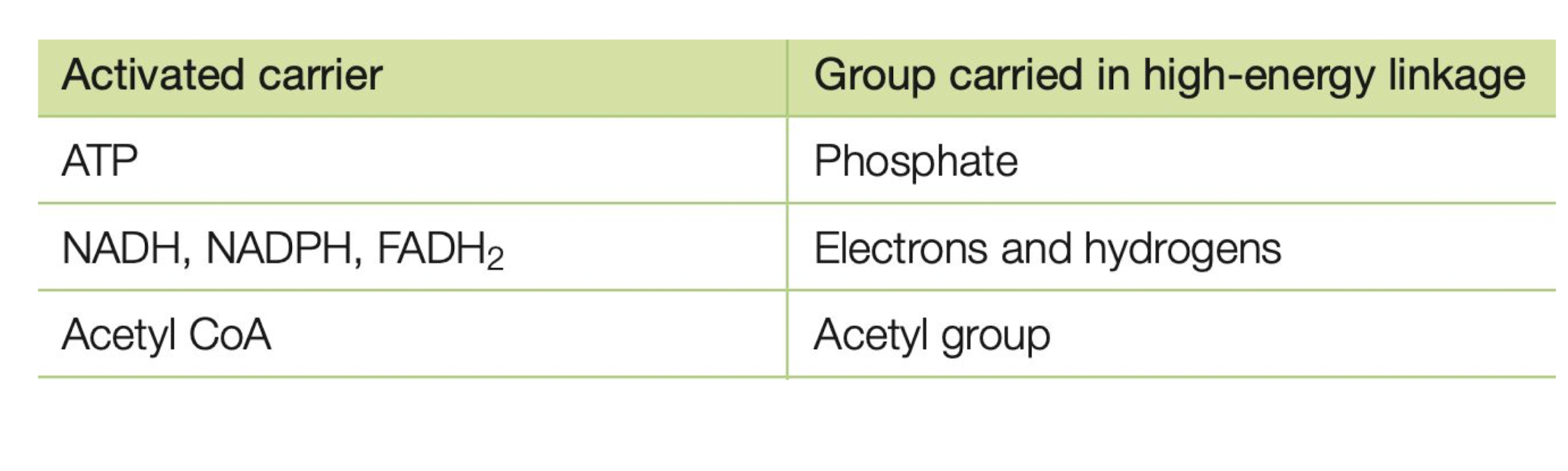
What are the main carrier molecules dealing with energy?
1. ATP
2. NADH
3. NADPH
4. Acetyl CoA
5. FADH2
10
New cards
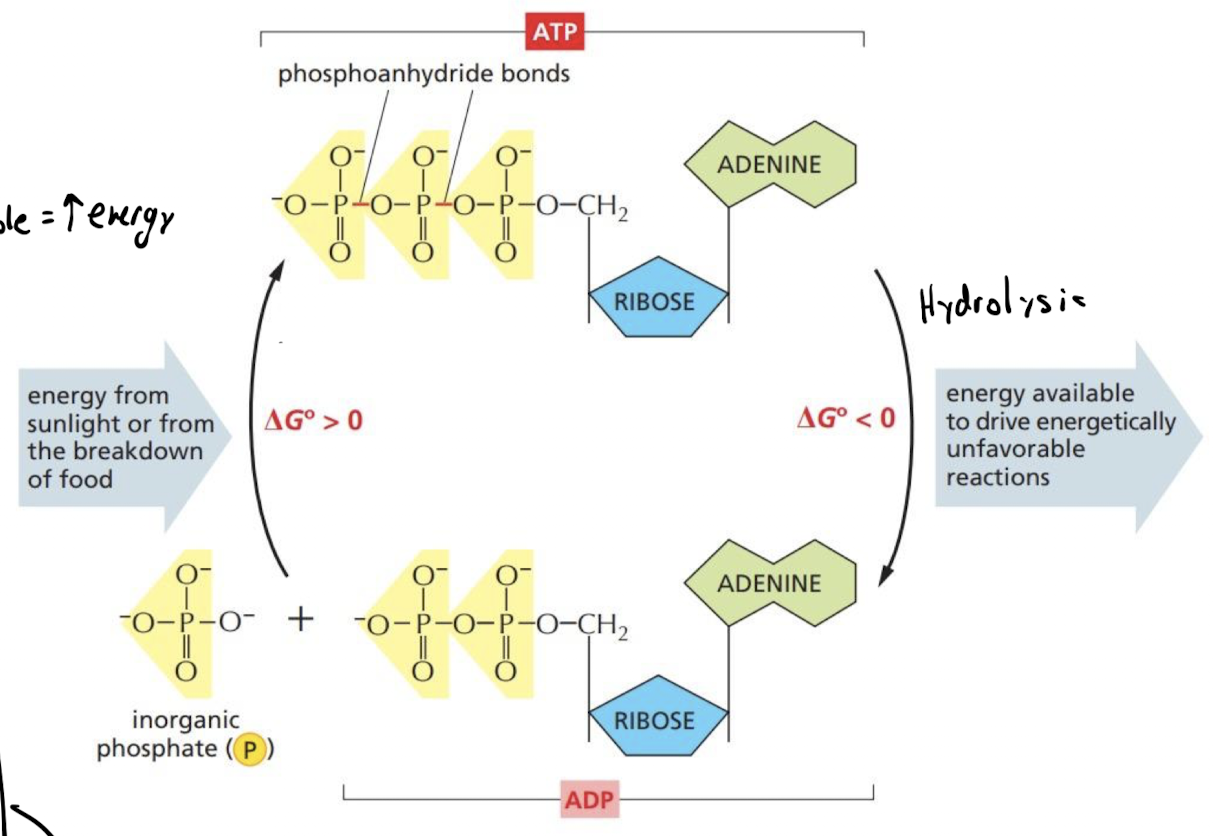
How does ATP store energy?
Covalent bonds b/w phosphate group and rest of molecule
11
New cards
How do NADH and NADPH store energy?
By carrying electrons held at high energy levels and hydrogen atoms
* NADH → reduced to add e-
* oxidizing agent for catabolism
* NAPDH → oxidized to add H+
* reducing agent for anabolism
* NADH → reduced to add e-
* oxidizing agent for catabolism
* NAPDH → oxidized to add H+
* reducing agent for anabolism
12
New cards
How does Acetyl CoA store energy?
Carries a transferable acetyl group
* used to add two carbon units to larger molecules
* used to add two carbon units to larger molecules
13
New cards
How does FADH2 store energy?
used like NADH in electron and proton transfer
14
New cards
Why do cells need controlled “steps” to release and harness chemical energy?
If it were to happen all at once the energy would be uncontrollably released too fast → similar to combustion
15
New cards
What is glycolysis?
The process of oxidizing sugars without using molecular oxygen, producing ATP
* occurs in cystol
* net gain of **2 ATP** and **2 Pyruvate** from **1 glucose**
* occurs in cystol
* net gain of **2 ATP** and **2 Pyruvate** from **1 glucose**
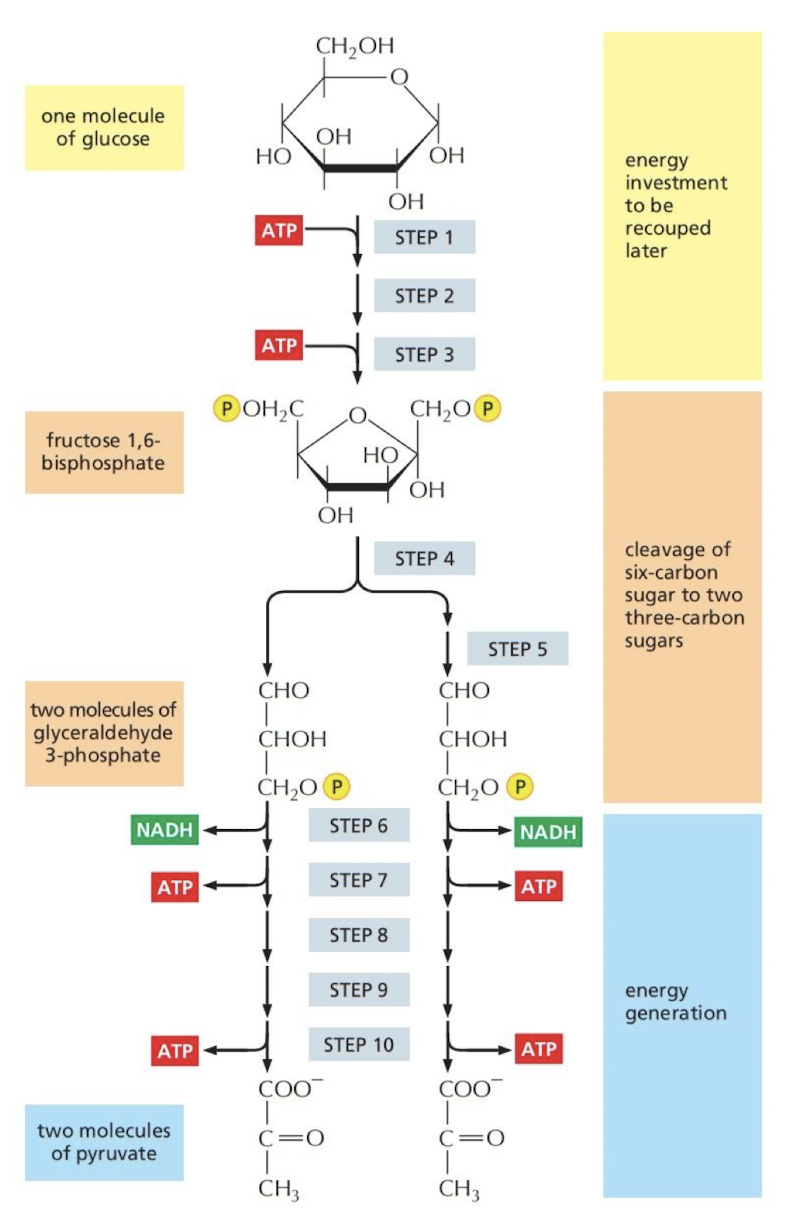
16
New cards
How is Acetyl CoA produced in cells?
both **pyruvate** and **fatty acids** are **degraded** to produce Acetyl CoA in the **mitochondria**
* 1 NADH and 1 FADH2 are also produced in this process
* 1 NADH and 1 FADH2 are also produced in this process
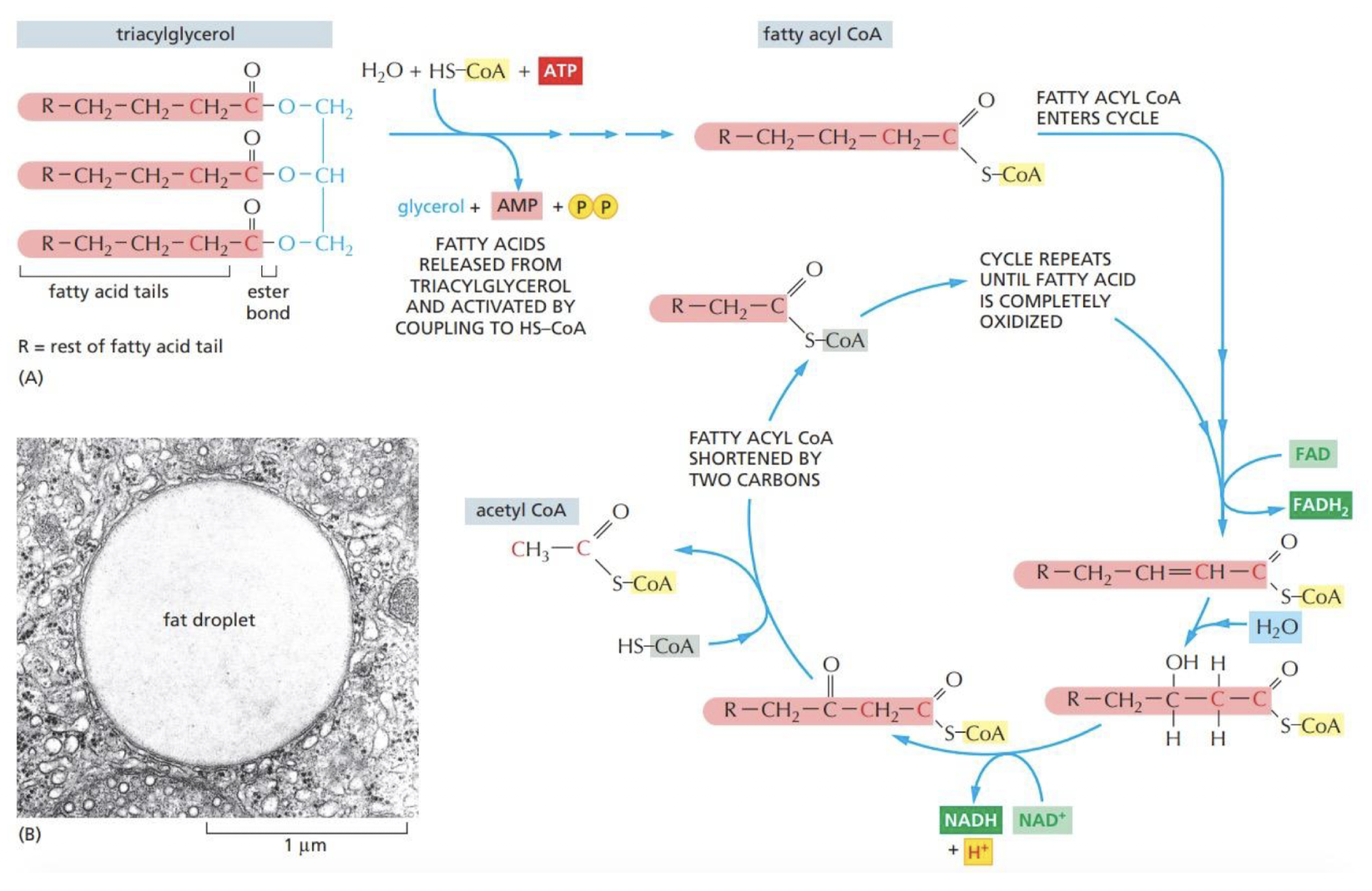
17
New cards
What is the citric acid cycle and what’s the main end result of the cycle?
1 turn of the cycle:
1 Acetyl CoA → 3 NADH + 1 GTP + 1 FADH + 2CO2
1 Acetyl CoA → 3 NADH + 1 GTP + 1 FADH + 2CO2
18
New cards
How does the electron transport chain continue the flow of energy in the cell?
The flow of e- from the citric acid cycle powers H+ pumps
* high H+ concentration within membrane of mitochondria generates a gradient
* The NADH dehydrogenase complex (Complex I) accepts electrons from NADH and transfer it to ubiquinone
* The cytochrome c reductase (Complex III) accepts electrons from ubiquinol and passes them on to cytochrome c
* The cytochrome c oxidase complex (Complex IV) accepts electrons one at a time from cytochrome c and passes them to molecular oxygen.
* high H+ concentration within membrane of mitochondria generates a gradient
* The NADH dehydrogenase complex (Complex I) accepts electrons from NADH and transfer it to ubiquinone
* The cytochrome c reductase (Complex III) accepts electrons from ubiquinol and passes them on to cytochrome c
* The cytochrome c oxidase complex (Complex IV) accepts electrons one at a time from cytochrome c and passes them to molecular oxygen.
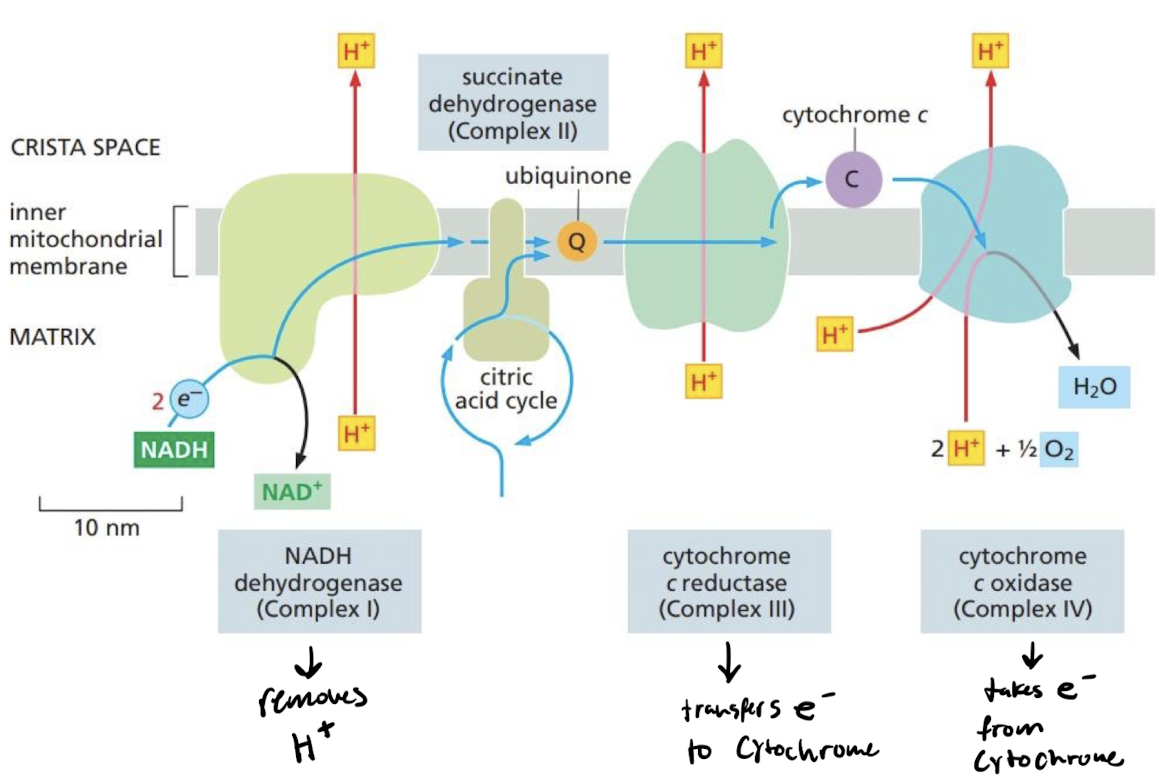
19
New cards
How does F-Type ATPase work to synthesize ATP?
flow of H+ moves its rotor stalk which adds phosphates to ADP, creating ATP
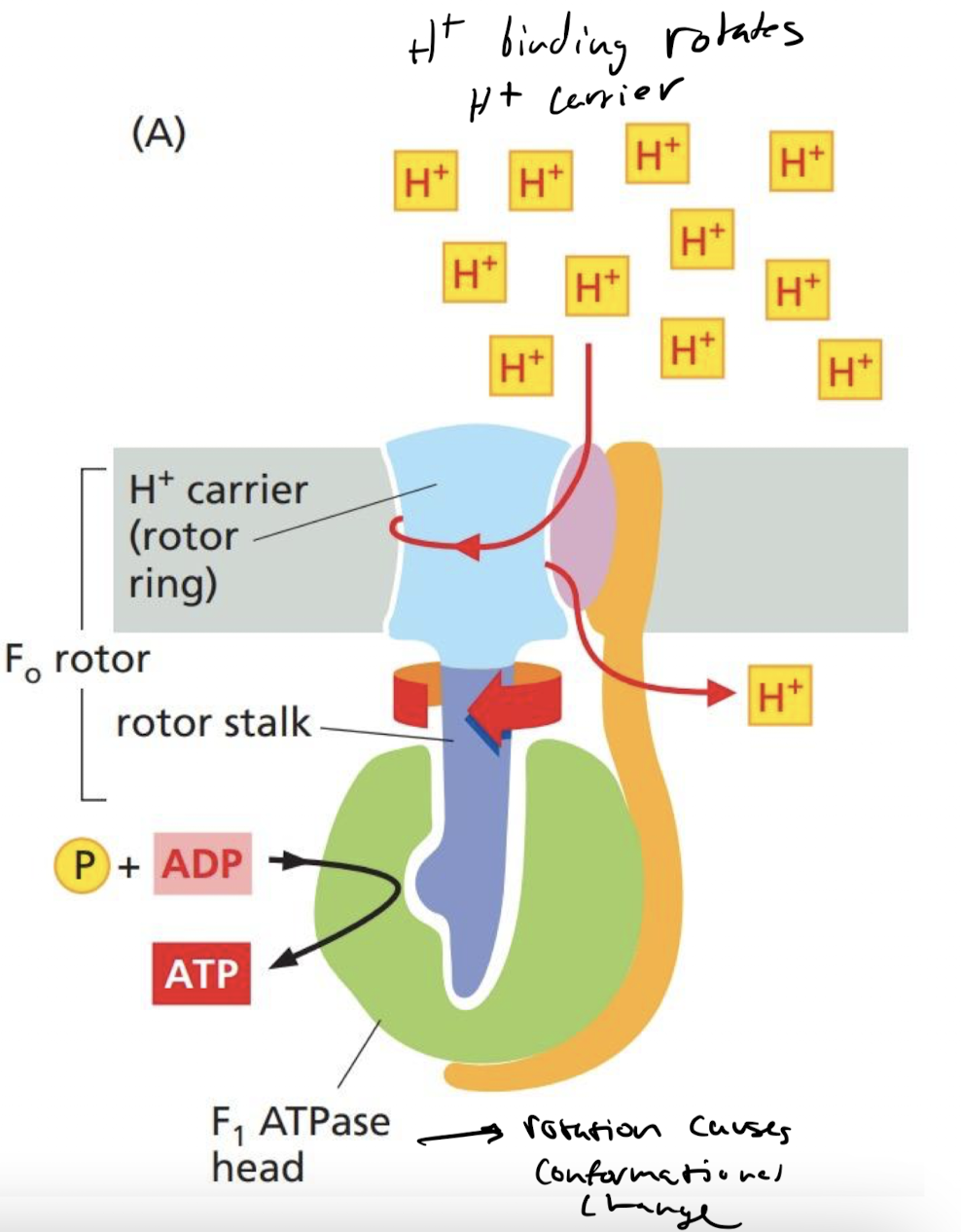
20
New cards
How does ATP production differ in anaerobic environments?
cells still have pyruvate and NADH
* NADH converted to NAD+
* pyruvate is converted to lactate/ethanol
* end result is ATP
* NADH converted to NAD+
* pyruvate is converted to lactate/ethanol
* end result is ATP
21
New cards
Full summary of ATP production
Acetyl CoA → citric acid cycle → electron transport chain → ATP synthase → ATP
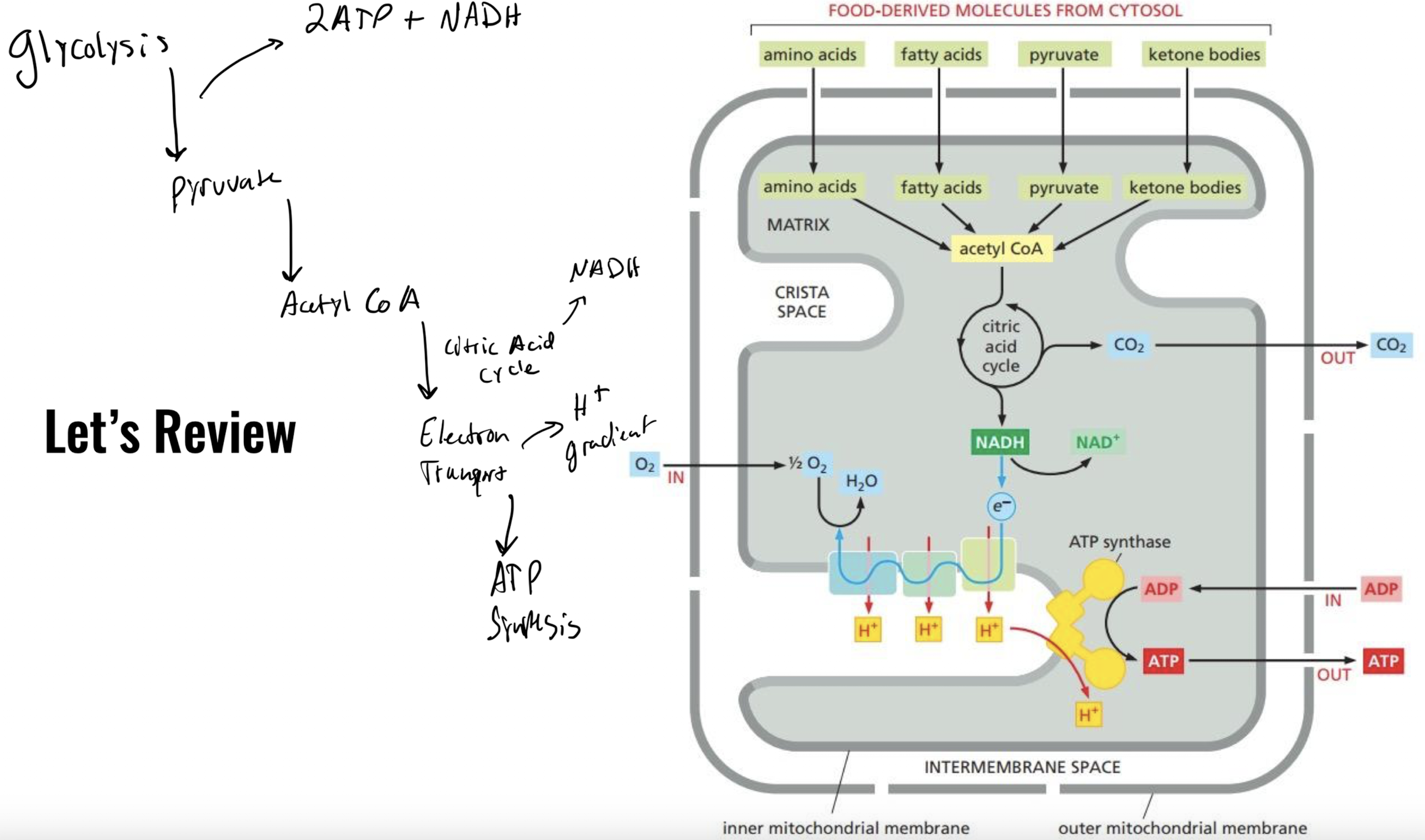
22
New cards
Which of the following represents an “activated” carrier molecule?
A. NADH
B. NAD+
C. NADP+
D. CoA
A. NADH
B. NAD+
C. NADP+
D. CoA
A. NADH
23
New cards
What is the end product of glycolysis in the cytoplasm of eukaryotic cells? How many carbon atoms does the molecule have?
a. Acetyl CoA; it has two carbon atoms attached to coenzyme A
b. Phosphoenolpyruvate; it has three carbon atoms
c. Glucose 6-phosphate; it has six carbon atoms
d. Pyruvate; it has three carbon atoms
e. Glyceraldehyde 3-phosphate; it has three carbon atoms
a. Acetyl CoA; it has two carbon atoms attached to coenzyme A
b. Phosphoenolpyruvate; it has three carbon atoms
c. Glucose 6-phosphate; it has six carbon atoms
d. Pyruvate; it has three carbon atoms
e. Glyceraldehyde 3-phosphate; it has three carbon atoms
d. Pyruvate; it has three carbon atoms
24
New cards
What processes is the cytoskeleton involved in?
1. interacting mechanically with each other
2. Interacting with their environment
3. Correct shape and proper internal structures
4. Changing the shape
5. Movement
6. rearranging internal components
7. Cell division
8. Adapting to change
25
New cards
What are actin filaments and how are they involved in the cytoskeleton?
* helical polymers made up of actin subunits
* most abundant in cortex (just below membrane)
* involved in…
* cell locomotion
* muscle contractions
* cytokinesis
* most abundant in cortex (just below membrane)
* involved in…
* cell locomotion
* muscle contractions
* cytokinesis
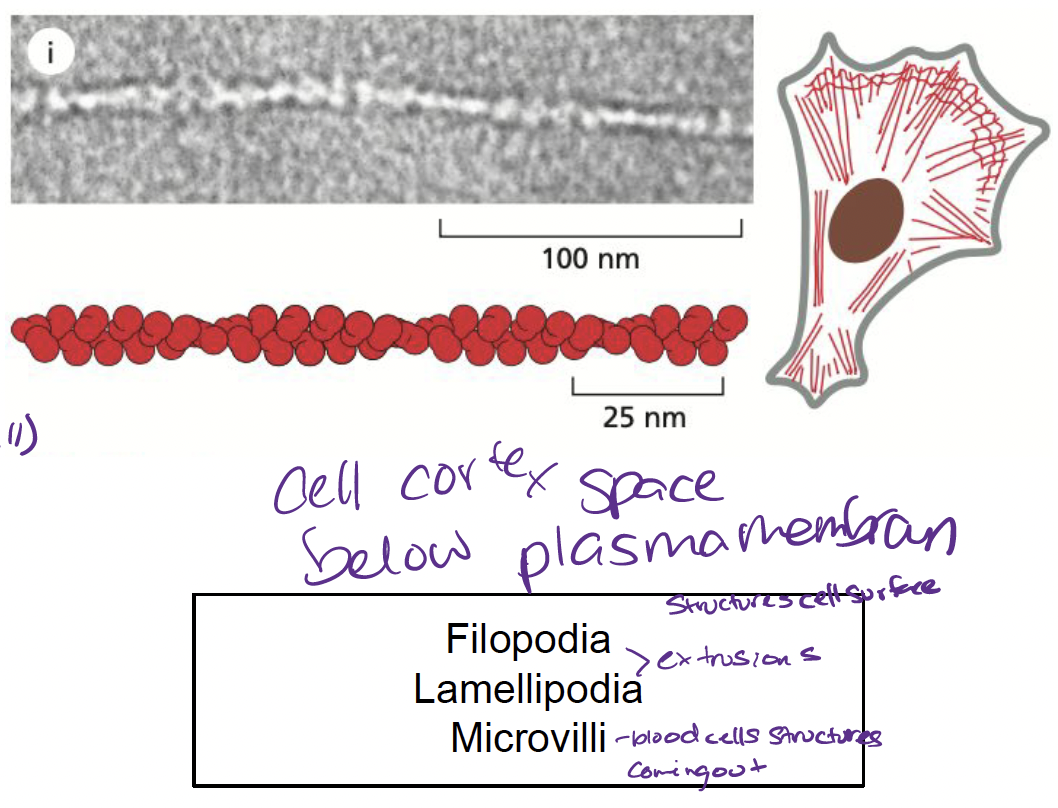
26
New cards
What are microtubules and how are they involved in the cytoskeleton?
* cylindrical polymers made up of tubulin protein subunit
* involved in…
* positioning organelles
* forming mitotic spindle to segregate chromosomes in cell division
* involved in…
* positioning organelles
* forming mitotic spindle to segregate chromosomes in cell division
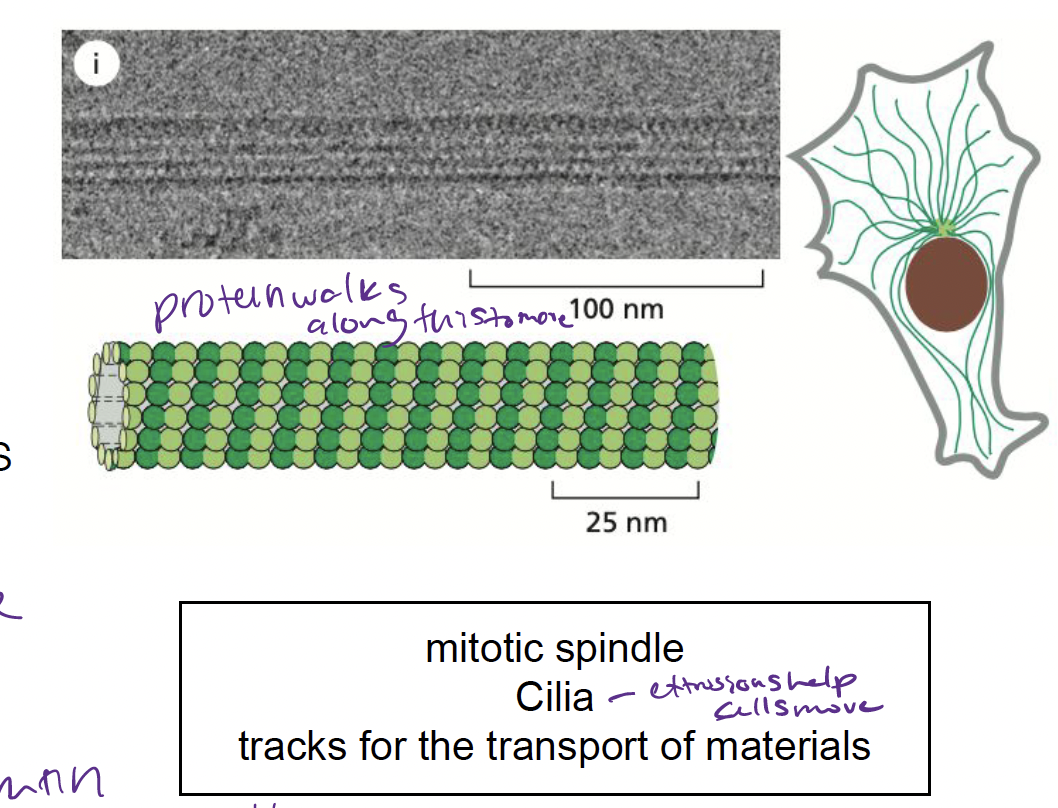
27
New cards
What are intermediate filatments?
made up of intermediate filament proteins
* provide mechanical strength
* provide mechanical strength
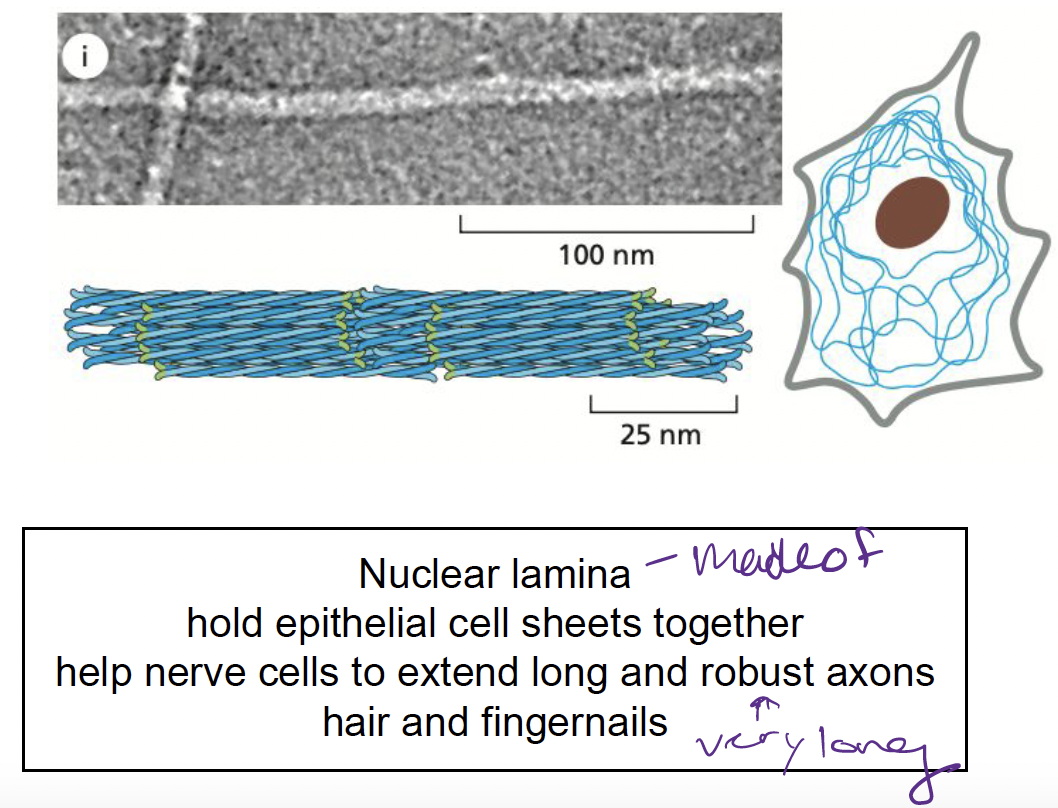
28
New cards
How does the cytoskeleton maintain cellular polarity in the epithelium?
* **Actin filaments** → form microvilli, anchor cells to each other via adhesion junctions
* **Intermediate filaments** → connect epithelial cells into a sturdy sheet, attaching to extracellular matrix
* **Microtubules** → provide global coordinate system for directing components inside cell
* **Intermediate filaments** → connect epithelial cells into a sturdy sheet, attaching to extracellular matrix
* **Microtubules** → provide global coordinate system for directing components inside cell
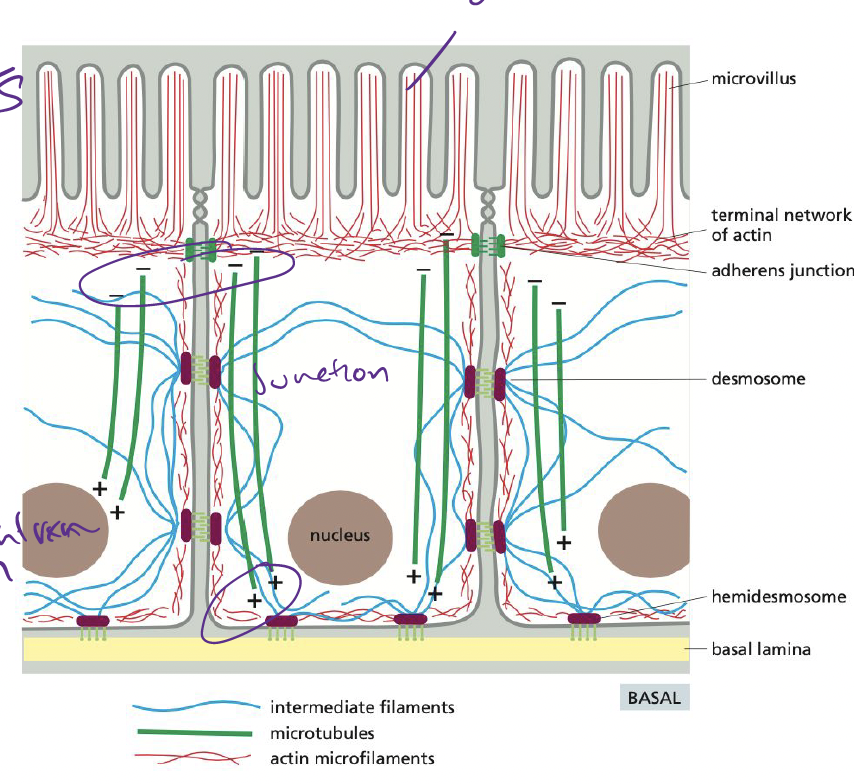
29
New cards
What are actin subunits called?
globular or G-actin
* assemble head-to-tail to form a right-handed helix forming F-actin
* subunits are asymmetrical → polar
* slower growing minus end
* faster growing plus end
* assemble head-to-tail to form a right-handed helix forming F-actin
* subunits are asymmetrical → polar
* slower growing minus end
* faster growing plus end

30
New cards
What is nucleation and what are its 3 phases?
Nucleation = actin subunits bind to one another to form nucleus, from which filament elongates with addition of more subunits
The lag phase → corresponds with time it takes for nucleation (initial subunits coming together)
The growth phase → occurs as subunits add onto and elongate the filament
The equilibrium phase → addition of new subunits and disassembly of subunits keeps fiber at constant size
The lag phase → corresponds with time it takes for nucleation (initial subunits coming together)
The growth phase → occurs as subunits add onto and elongate the filament
The equilibrium phase → addition of new subunits and disassembly of subunits keeps fiber at constant size
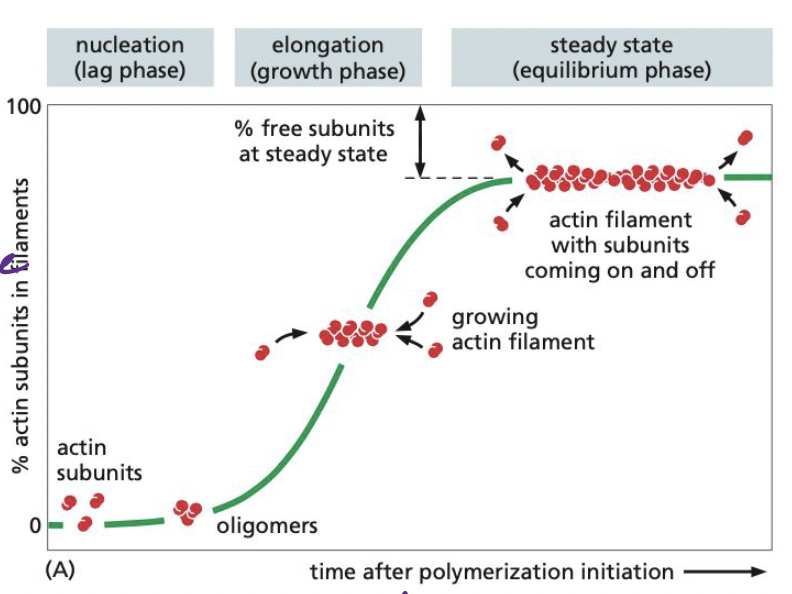
31
New cards
In nucleation, what is critical concentration?
Its the concentration level of actin where the equilibrium phase of nucleation happens
32
New cards
How can cells skip the “lag phase”?
preexisting acting fragments act as seeds to allow elongation to happen faster when needed
* enzymes catalyze at specific sites determining where new actin filaments are formed
* enzymes catalyze at specific sites determining where new actin filaments are formed
33
New cards
What usually catalyzes actin nucleation?
Arp2/3 complex
* requires nucleation-promoting factor activity to mediate nucleation catalysis (binds to minus end)
Or the formins
* requires nucleation-promoting factor activity to mediate nucleation catalysis (binds to minus end)
Or the formins
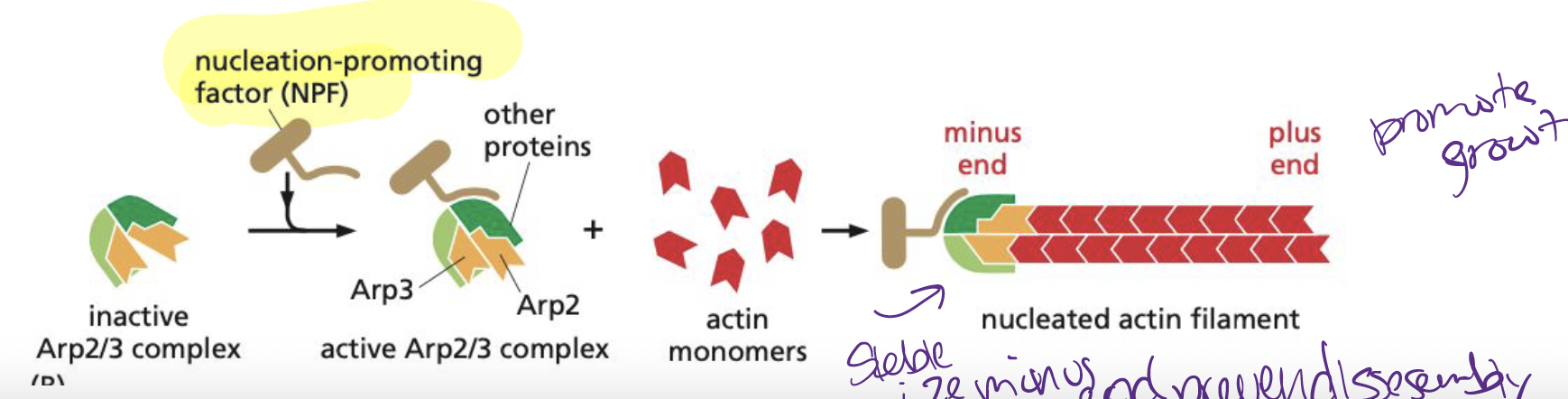
34
New cards
when is the Arp2/3 complex most efficient at catalyzing nucleation?
When the complex is bond to a preexisting filament (activates branched array of filaments)
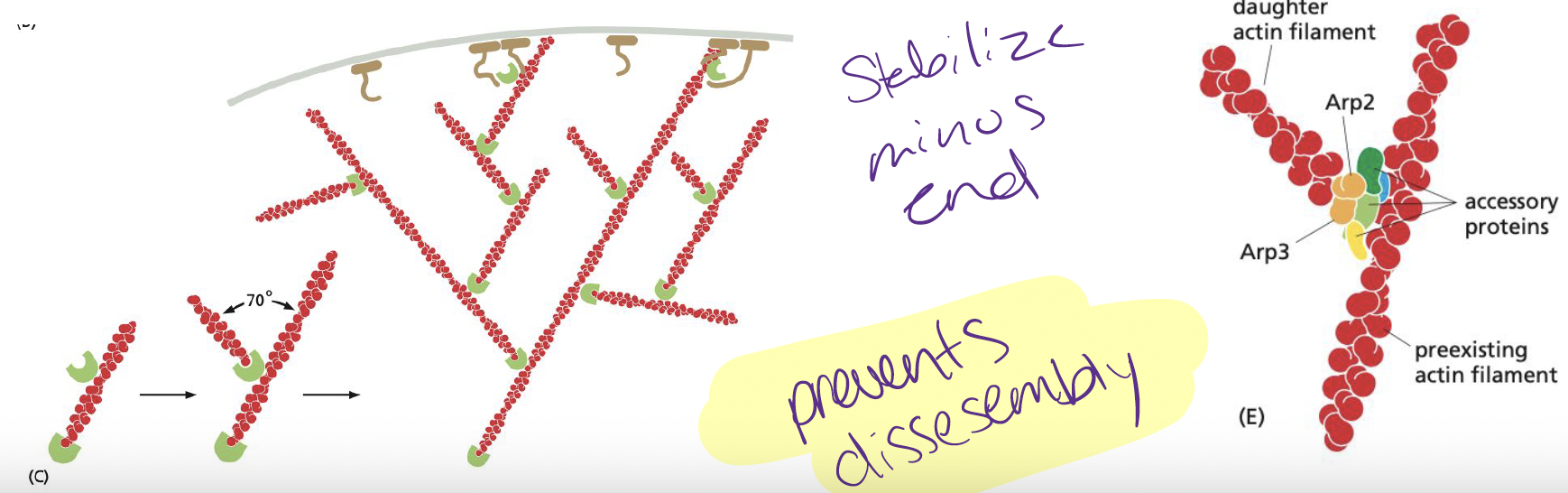
35
New cards
What are formins?
dimeric proteins that nucleate the growth of unbranched filaments
* each formin subunit has binding site on monomeric actin → rapid plus end
* also accelerate filament growth
* each formin subunit has binding site on monomeric actin → rapid plus end
* also accelerate filament growth
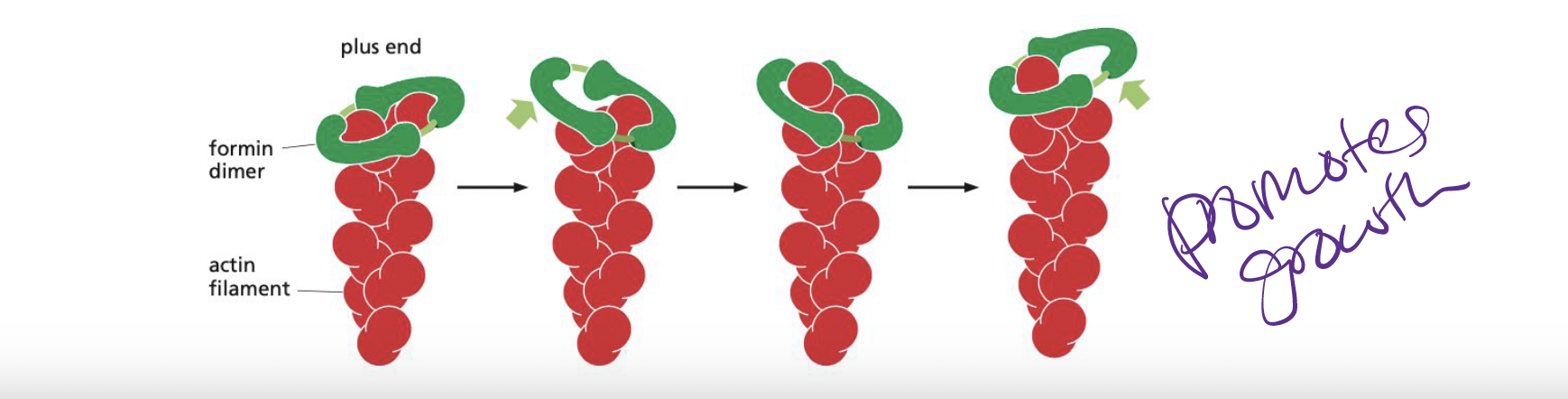
36
New cards
What is profilin?
binds to actin filament and enhances its growth
* proflin maintains a large pool of actin monomers poised for polymerization
* binding sites for proflin are present in many formin proteins, as well as in many NPFs
* proflin maintains a large pool of actin monomers poised for polymerization
* binding sites for proflin are present in many formin proteins, as well as in many NPFs
37
New cards
What does the profilin-actin complex do?
binds to the plus end of an actin filament, end, a conformational change in actin reduces its affnity for proflin and the proflin falls off, leaving the actin flament one subunit longer.
38
New cards
What are filopodia?
essentially 1-D, bundled parallel actin filaments
* help cells sense for environmental cues
* helps cells in cell migration
* help cells sense for environmental cues
* helps cells in cell migration
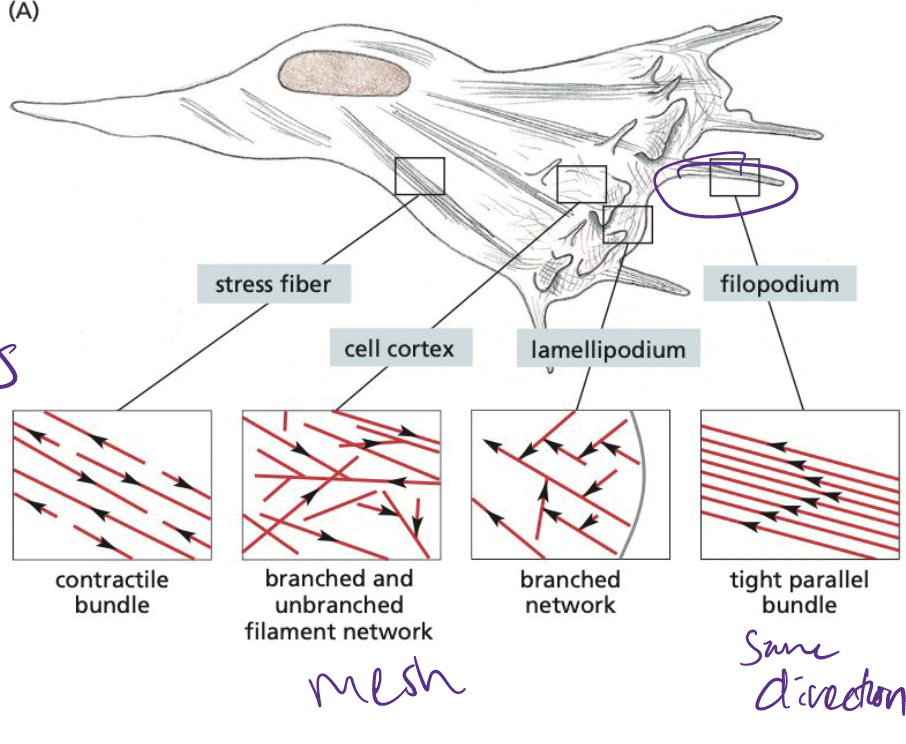
39
New cards
What are Lamellipodia?
2-D, sheet like structures with a cross-linked mesh of actin flaments, forming a plane parallel to the solid substratum.
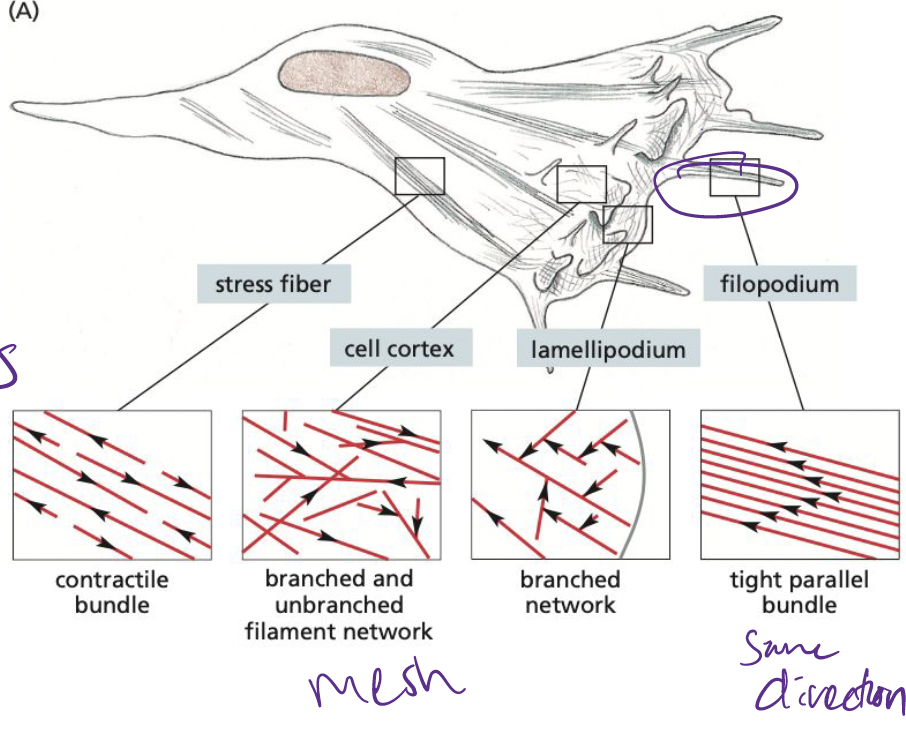
40
New cards
What is myosin II?
elongated protein formed from two heavy chains and two copies of each of two light chains.
* each chain has globular head at n-terminus w/ force-generating machinery
* majority of length is a long alpha helix → mediates heavy-chain dimerization
* each chain has globular head at n-terminus w/ force-generating machinery
* majority of length is a long alpha helix → mediates heavy-chain dimerization
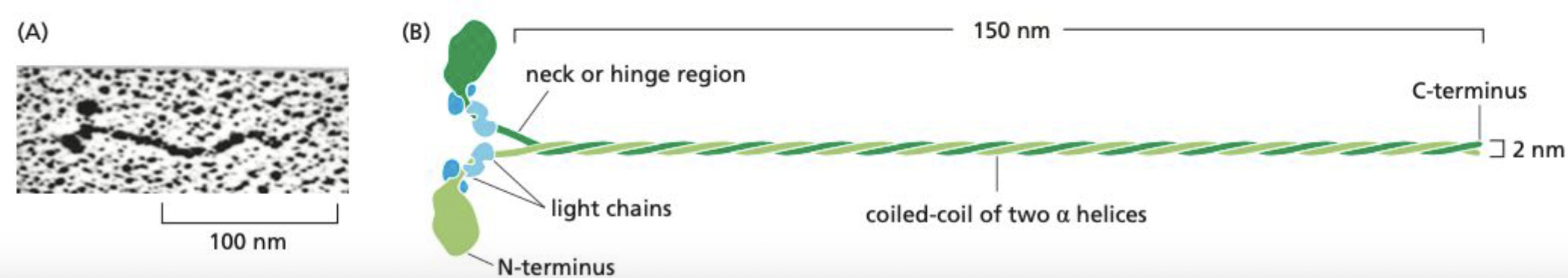
41
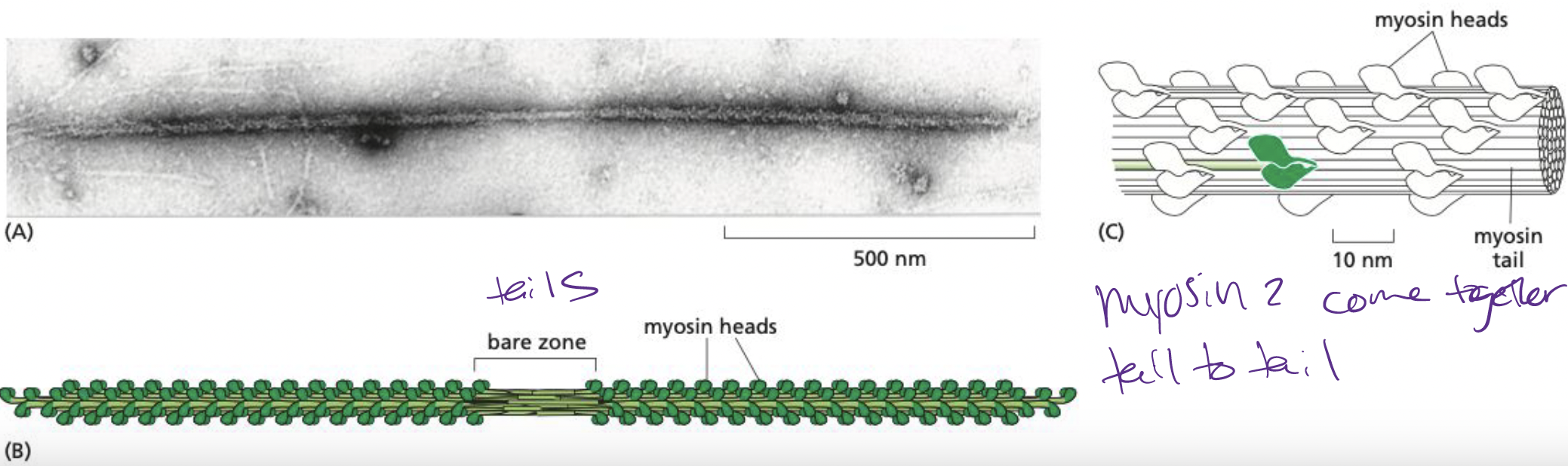
New cards
How does myosin II come together?
Tail-tail interactions form large, bipolar thick filaments that have several hundred myosin heads, oriented in opposite directions at the two ends of the thick filament.
* each myosin head binds to and hydrolyzes ATP to walk towards plus end of actin filament
* each myosin head binds to and hydrolyzes ATP to walk towards plus end of actin filament
42
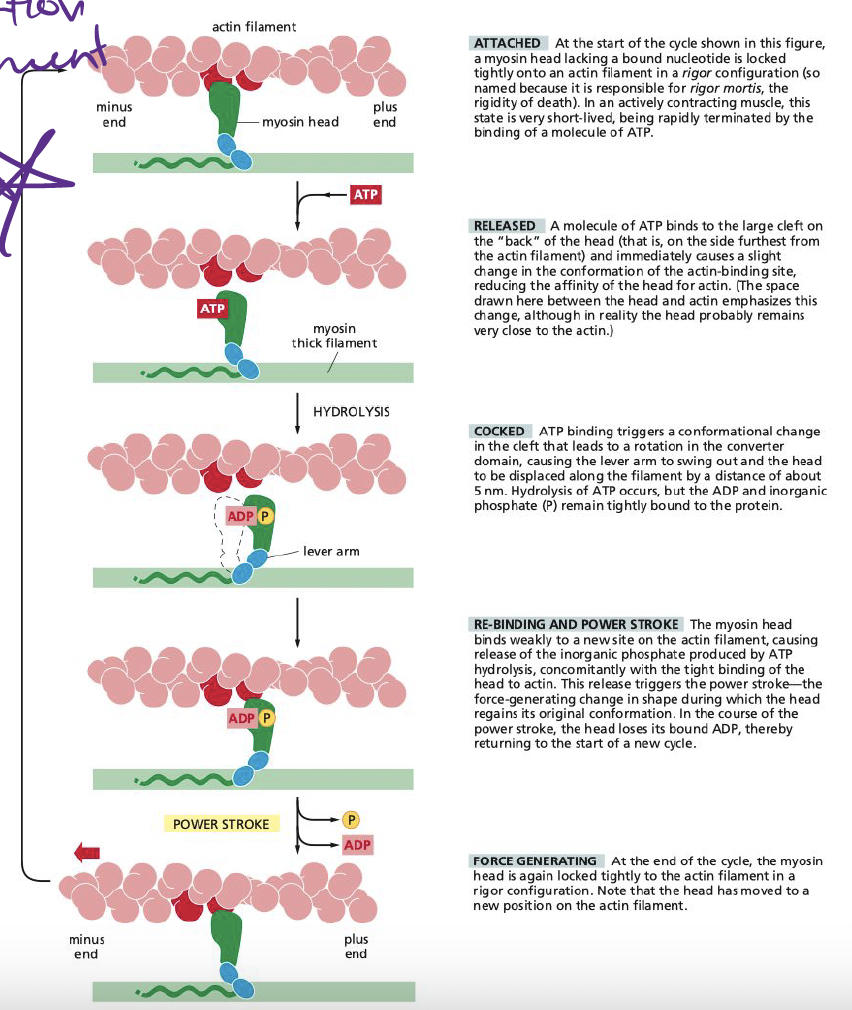
New cards
How does myosin generate force?
Binding of ATP to myosin alters conformation of protein
* coupled to changes in binding affinity for actin
* allows for myosin head to release grip on actin and grab it at another point to allow it to move
* allows muscles to contract
* coupled to changes in binding affinity for actin
* allows for myosin head to release grip on actin and grab it at another point to allow it to move
* allows muscles to contract
43
New cards
What are sarcomeres?
Small contractile units of muscle tissue
* composed of actin, anchored at plus end to Z-disk
* capped minus end extend toward middle of sarcomere, overlapping thick filaments
* composed of actin, anchored at plus end to Z-disk
* capped minus end extend toward middle of sarcomere, overlapping thick filaments
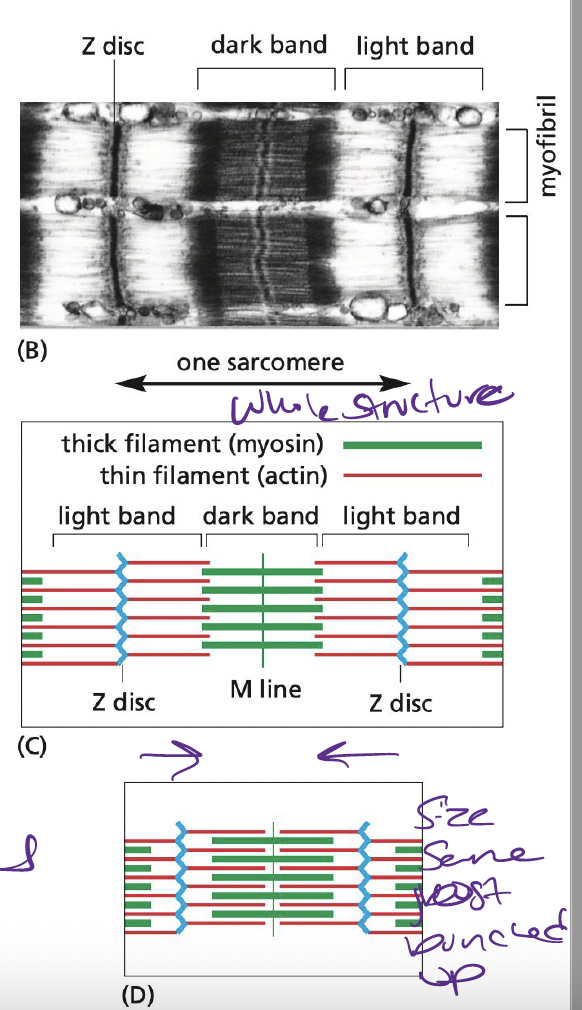
44
New cards
What initiates muscle contraction?
AP from nerve transfers through T tubulin (smooth ER) to cause a sudden rise in cystolic Ca2+ concentration
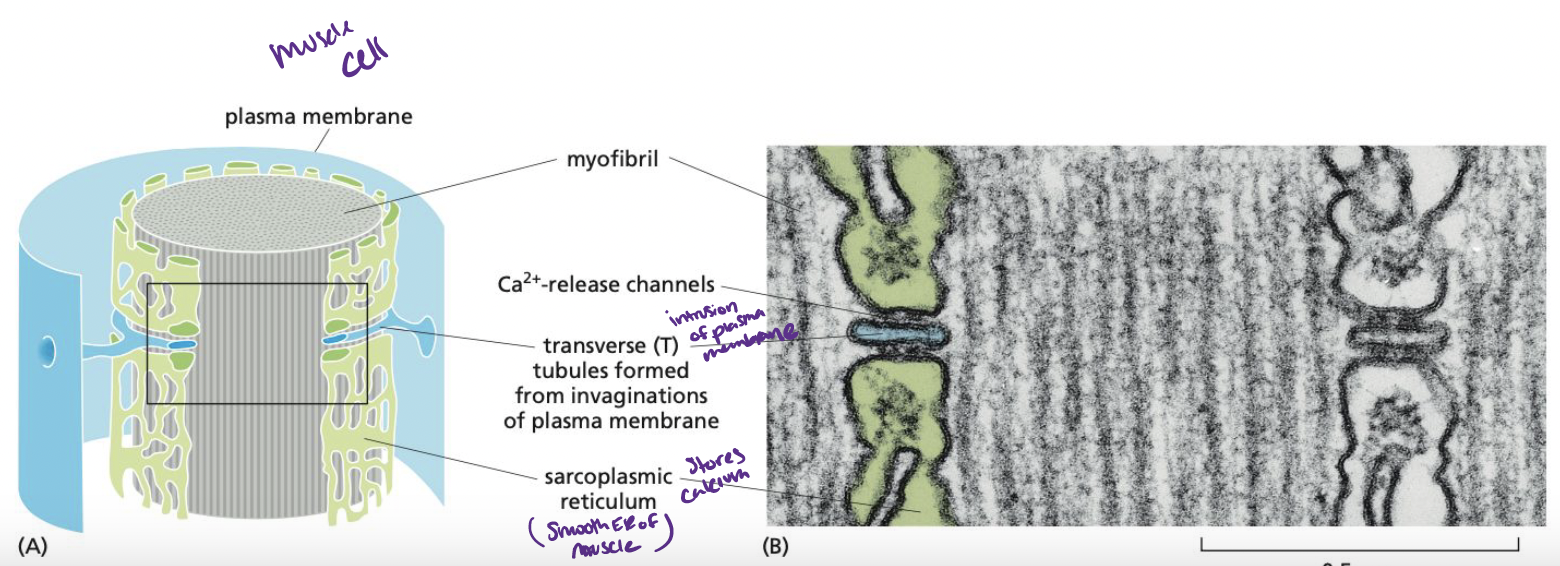
45
New cards
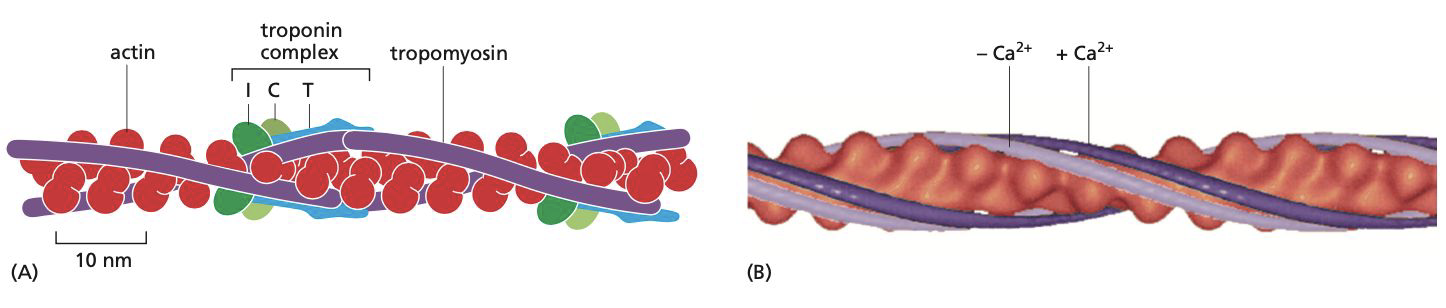
How is troponin and tropomyosin involved in muscle contraction?
**Tropomyosin** = long protein that attaches itself to the groove of the actin filament
**Troponin** = complex made up of three smaller proteins: troponin T(tropomyosin-binding), I (inhibits contraction), and C (binds to Ca2+).
**Relaxed muscle** = the troponin I-T complex pulls the tropomyosin into a position that prevents the binding of myosin heads.
**Muscle contraction** = concentration of calcium ions increases, troponin C causes troponin I to let go of actin. This allows the tropomyosin molecules to return to their normal position, allowing the myosin heads to move along the actin filaments, leading to muscle contraction.
**Troponin** = complex made up of three smaller proteins: troponin T(tropomyosin-binding), I (inhibits contraction), and C (binds to Ca2+).
**Relaxed muscle** = the troponin I-T complex pulls the tropomyosin into a position that prevents the binding of myosin heads.
**Muscle contraction** = concentration of calcium ions increases, troponin C causes troponin I to let go of actin. This allows the tropomyosin molecules to return to their normal position, allowing the myosin heads to move along the actin filaments, leading to muscle contraction.
46
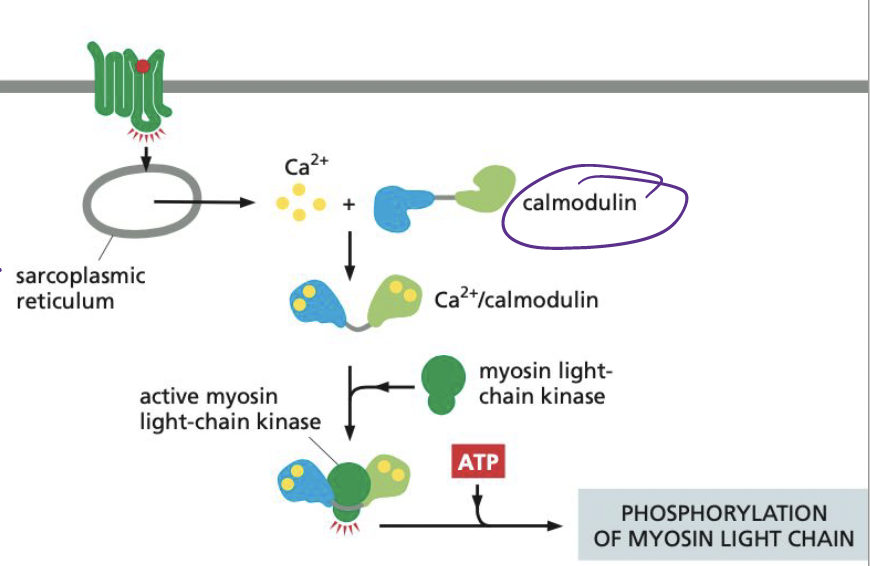
New cards
What does calmodulin do when bound to Ca2+?
Activates myosin light-chain kinase (MLCK), inducing the phosphorylation of smooth muscle myosin on one of its two light chains.
* allows for myosin-actin interaction → contraction
* allows for myosin-actin interaction → contraction
47
New cards
Which cytoskeletal filament is abundant in an animal cell
nucleus?
a. Actin filaments
B. Microtubules
C. Calmodulin
D. Intermediate filaments
nucleus?
a. Actin filaments
B. Microtubules
C. Calmodulin
D. Intermediate filaments
D. Intermediate filaments
48
New cards
In the polymerization in vitro of actin filaments and microtubules from their subunits, what does the "lag phase" correspond to?
A. Nucleation
B. Reaching steady state
C. Nucleotide exchange
D. ATP or GTP hydrolysis
E. Treadmilling
A. Nucleation
B. Reaching steady state
C. Nucleotide exchange
D. ATP or GTP hydrolysis
E. Treadmilling
A. Nucleation
49
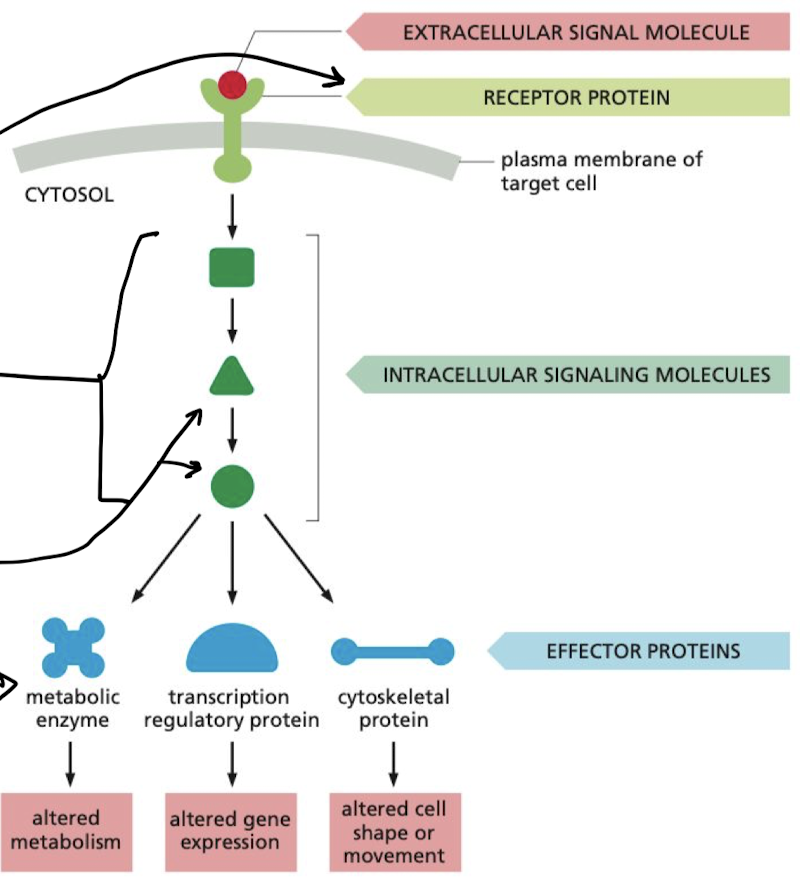
New cards
What is the flow of a signal in a simple intracellular signaling pathway?
extracellular signal molecule → receptor protein → intracellular signaling molecules → effector proteins
50
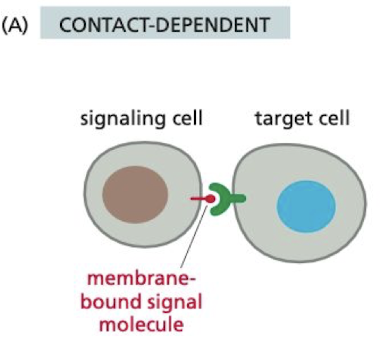
New cards
What is contact-dependent signaling?
requires cells to be in direct membrane-membrane contact to signal
* important in immune responses
* important in immune responses
51
New cards
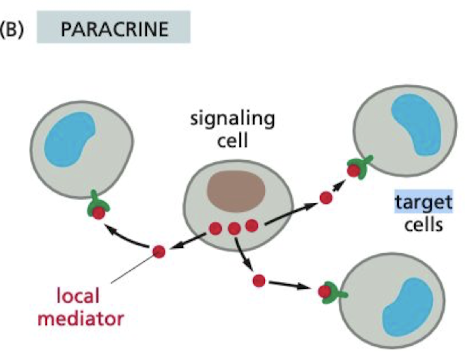
Whats paracrine cell-signaling?
signaling cell only acts on cells in the immediate area
* autocrine is when the cell is signaling to itself
* autocrine is when the cell is signaling to itself
52
New cards
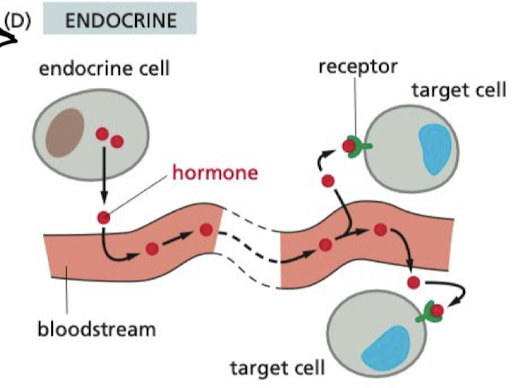
What is endocrine signaling?
hormones are secreted through the blood stream to impact cells all across the body
53
New cards
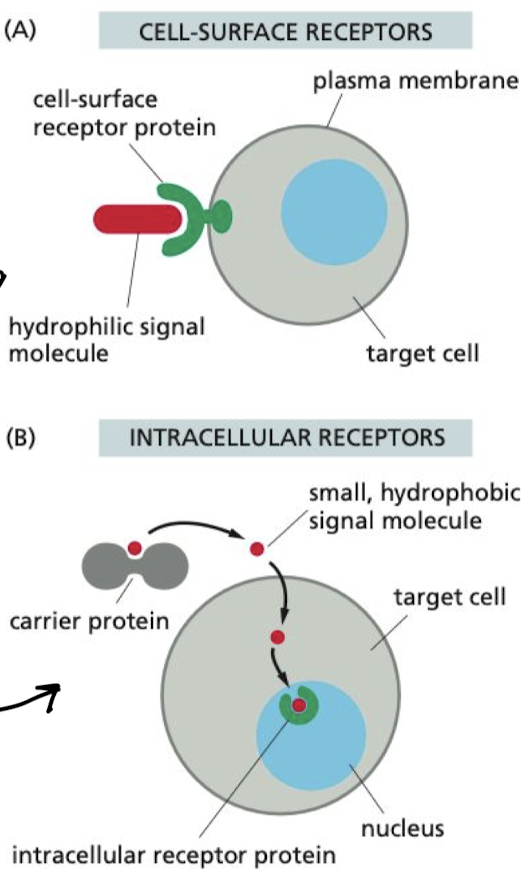
What are the two types of cell receptors?
1. Cell-surface receptors
1. Binds to external signal molecule, propagates signal inside of cell
2. Intracellular receptors
1. carrier protein carries signal molecules to membrane, where they diffuse and bind to receptor
54
New cards
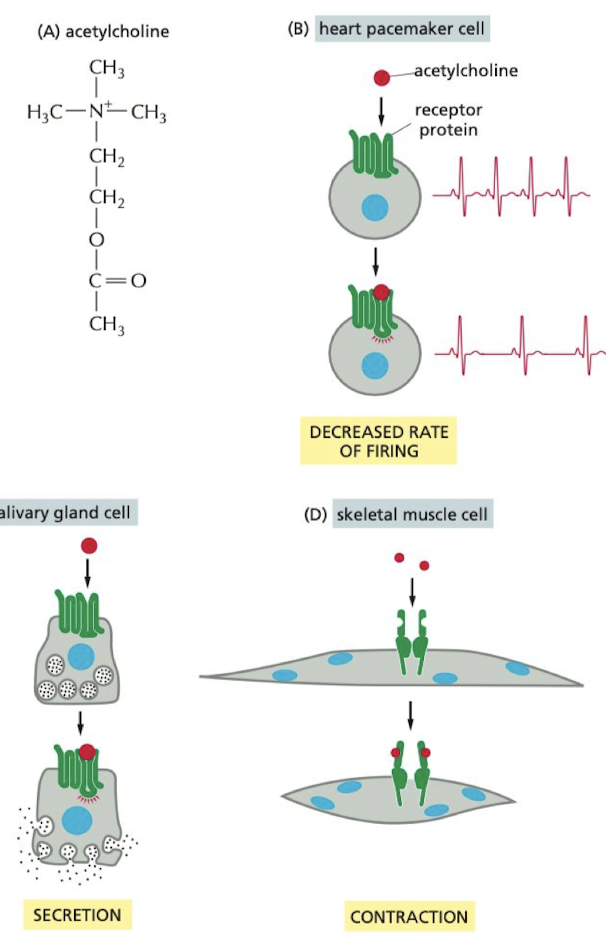
What are the different impacts of acetylcholine on the body?
1. pacemaker cell → decreases HR
2. salivary gland → increases saliva production
3. skeletal muscle cell → causes contraction
55
New cards
What are the 3 major classes of cell-surface receptors?
1. Ion-channel-coupled receptors
2. G-protein coupled receptors
3. Enzyme-coupled receptors
56
New cards
How do Ion-channel-coupled receptors work?
Involved in rapid synaptic signaling b/w nerve cells and other electrically excitable cells
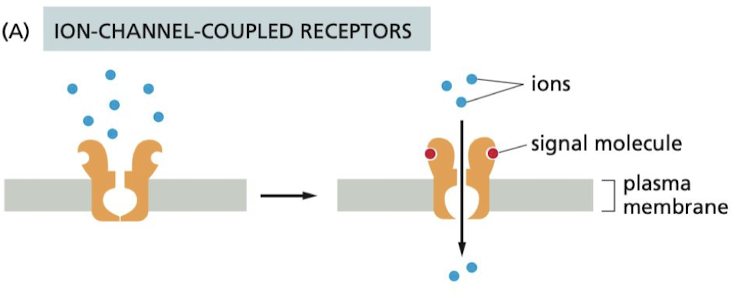
57
New cards
How do G-protein coupled receptors work?
controls the activity of another protein on the cell membrane, typically enzyme or ion channel.
* Activation of the target protein can change concentration or permeability of one or more small intracellular signaling molecules
* Activation of the target protein can change concentration or permeability of one or more small intracellular signaling molecules
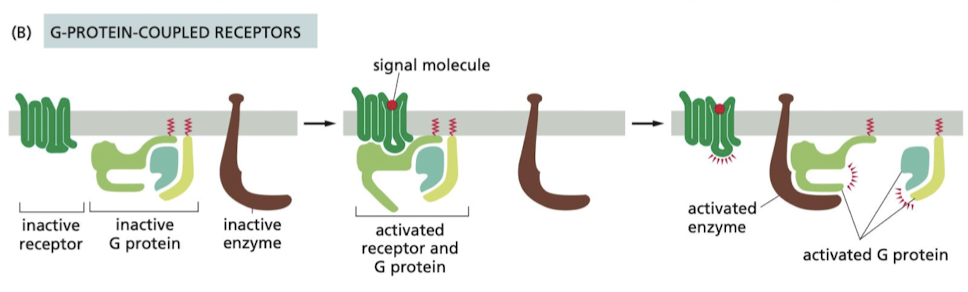
58
New cards
How do enzyme-coupled receptors work?
They either…
1. Function as an enzyme
2. Associate directly w/ enzymes they activate
* Majority of them are either protein kinases or associated with protein kinases
* many activated by ligands promoting dimerization
1. Function as an enzyme
2. Associate directly w/ enzymes they activate
* Majority of them are either protein kinases or associated with protein kinases
* many activated by ligands promoting dimerization
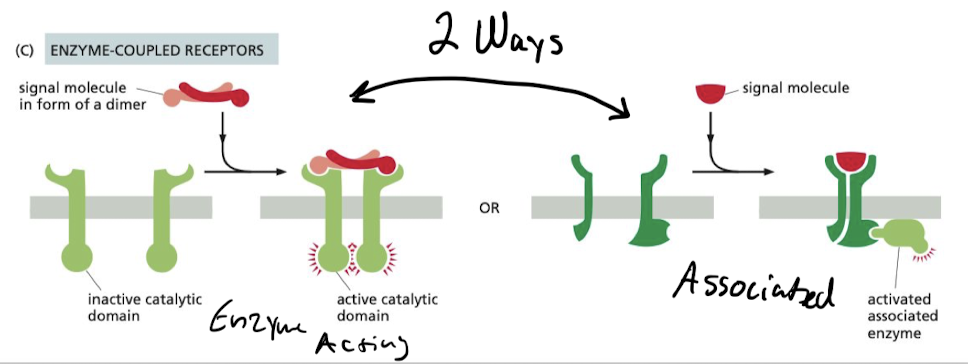
59
New cards
What are second messengers
Small molecules generated when receptor is activated. Profuse away from source to spread signal to rest of cell
* ex) cAMP and Ca2+
* ex) cAMP and Ca2+
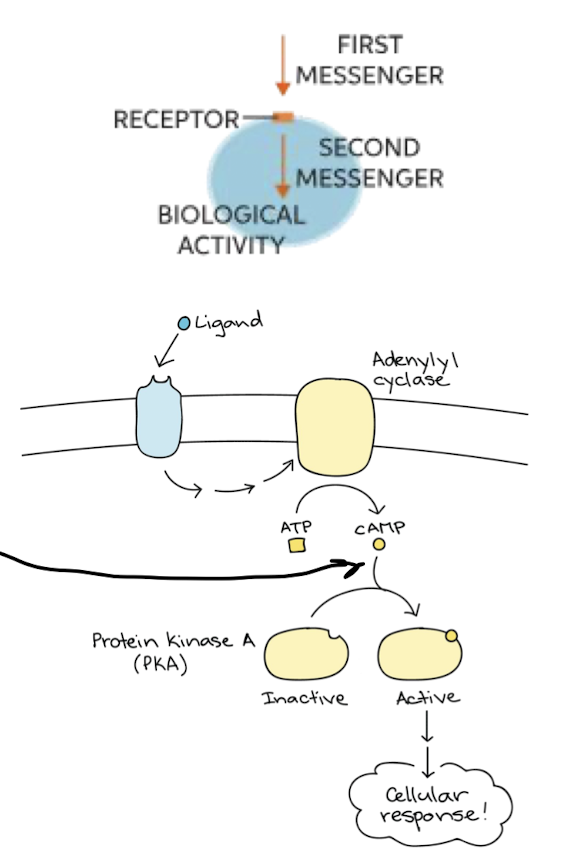
60
New cards
Most intracellular signaling molecules are proteins that behave like ___________.
molecular switches
61
New cards
What are the 2 types of molecular switches
1. phosphorylation-controlled switches
1. phosphorylation of the protein either activates or inactivates the protein
2. GTP-Binding proteins
1. when GTP is bound, protein switches b/w two conformations:
2. **on** when **GTP bound**
3. **off** when **GTP** **unbound**
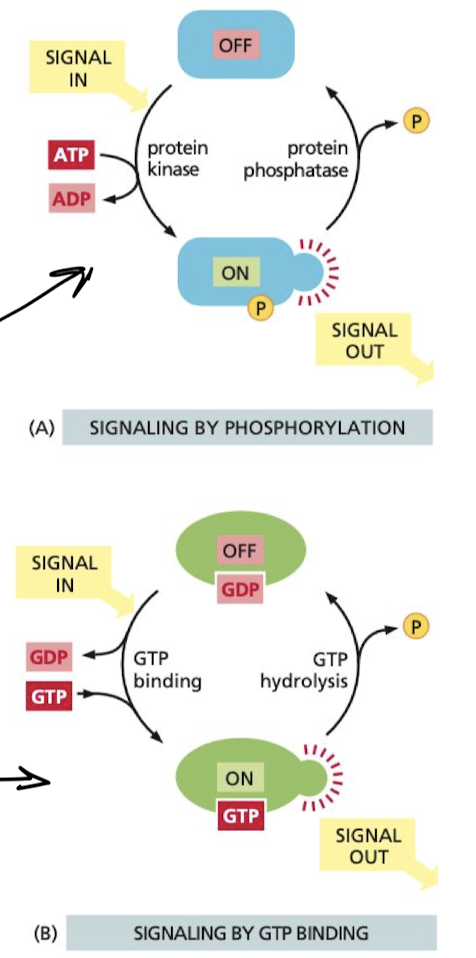
62
New cards
What increases and decreases the rate of GTP binding in GTP-Binding proteins?
GTPase-activating proteins (GAPs)
* increases rate of GTP hydrolysis turning it off faster
Guanine nucleotide exchange factors (GEFs)
* promote release of bound GTP, allowing new GTP to bind turning it on faster
* increases rate of GTP hydrolysis turning it off faster
Guanine nucleotide exchange factors (GEFs)
* promote release of bound GTP, allowing new GTP to bind turning it on faster
63
New cards
how do our cells increase the specificity of interacting cell signaling molecules, reducing background noise?
by localizing the molecules to the same part of the cell
64
New cards
What do scaffold proteins do?
corrals groups of interacting signaling proteins into complexes in anticipation of receiving a signal.
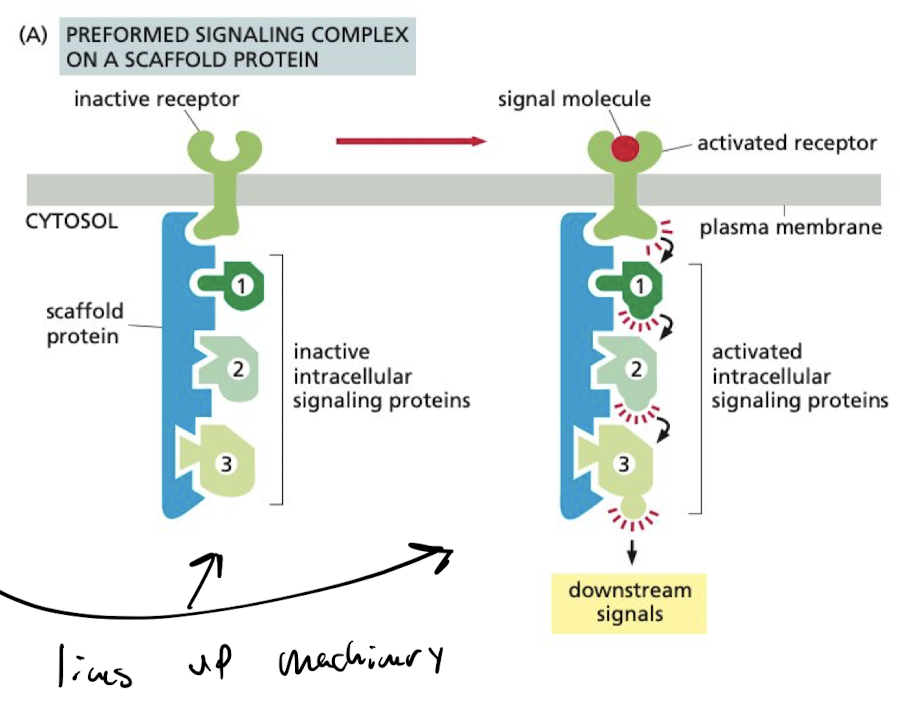
65
New cards
What makes the cell’s response to stimuli faster?
If the target proteins are already present, all the cell has to do is change the conformation which makes it fast.
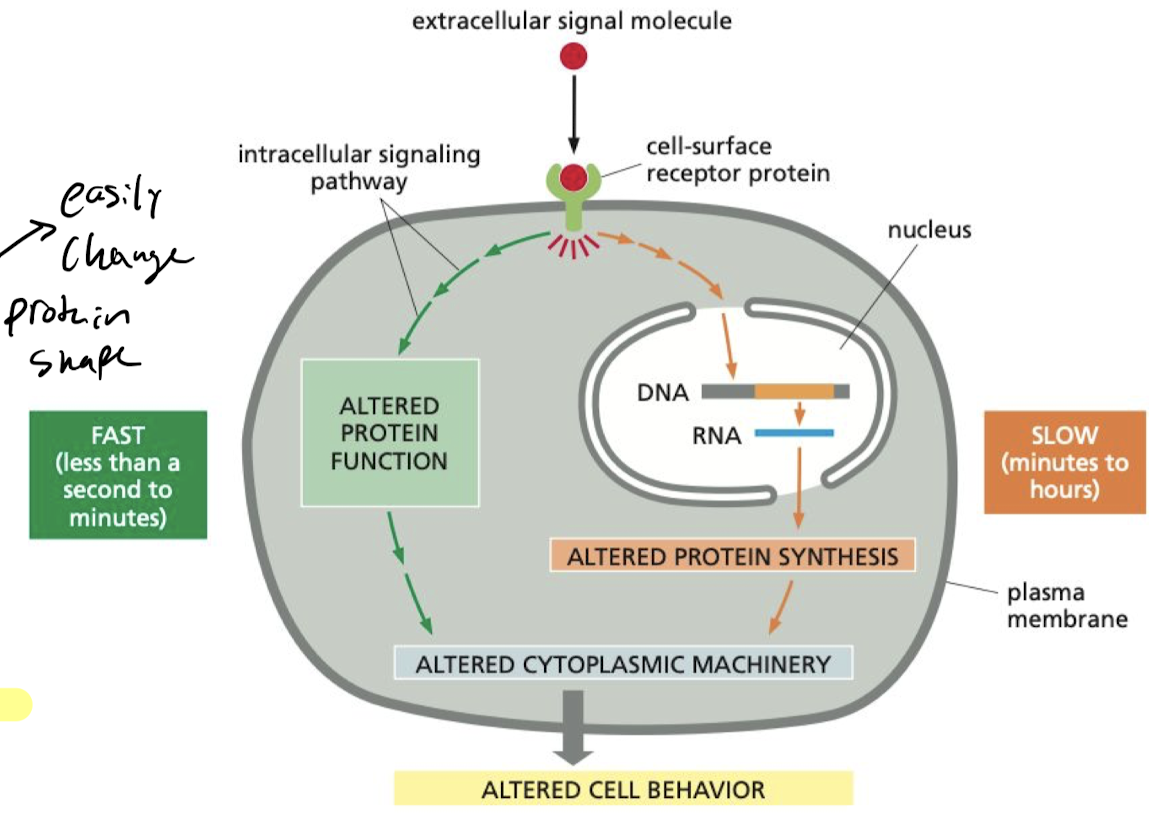
66
New cards
What makes the cell’s response to stimuli slower?
If the target proteins have not yet been synthesized, then the cell has to wait for gene expression to build the proteins it needs and shape them, making it slow
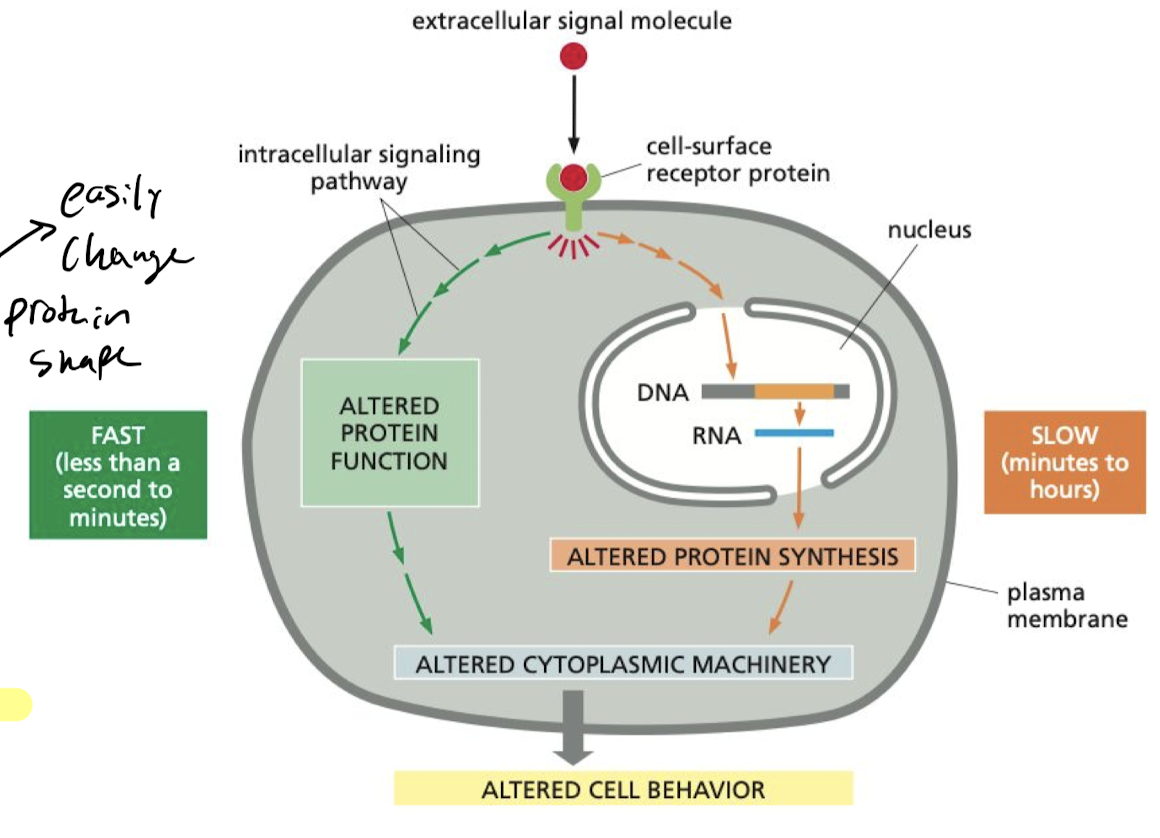
67
New cards
What is adaptation/desensitization in cell signaling?
Processes that allow cells to respond to changes in the strength of an input signal.
1. Negative Feedback
2. Activation of slow parallel pathway to slow response
3. Receptor inactivation
1. Activated receptor shuts itself off
2. Sequestration (endocytosis)
3. Degradation (exocytosis)
1. Negative Feedback
2. Activation of slow parallel pathway to slow response
3. Receptor inactivation
1. Activated receptor shuts itself off
2. Sequestration (endocytosis)
3. Degradation (exocytosis)
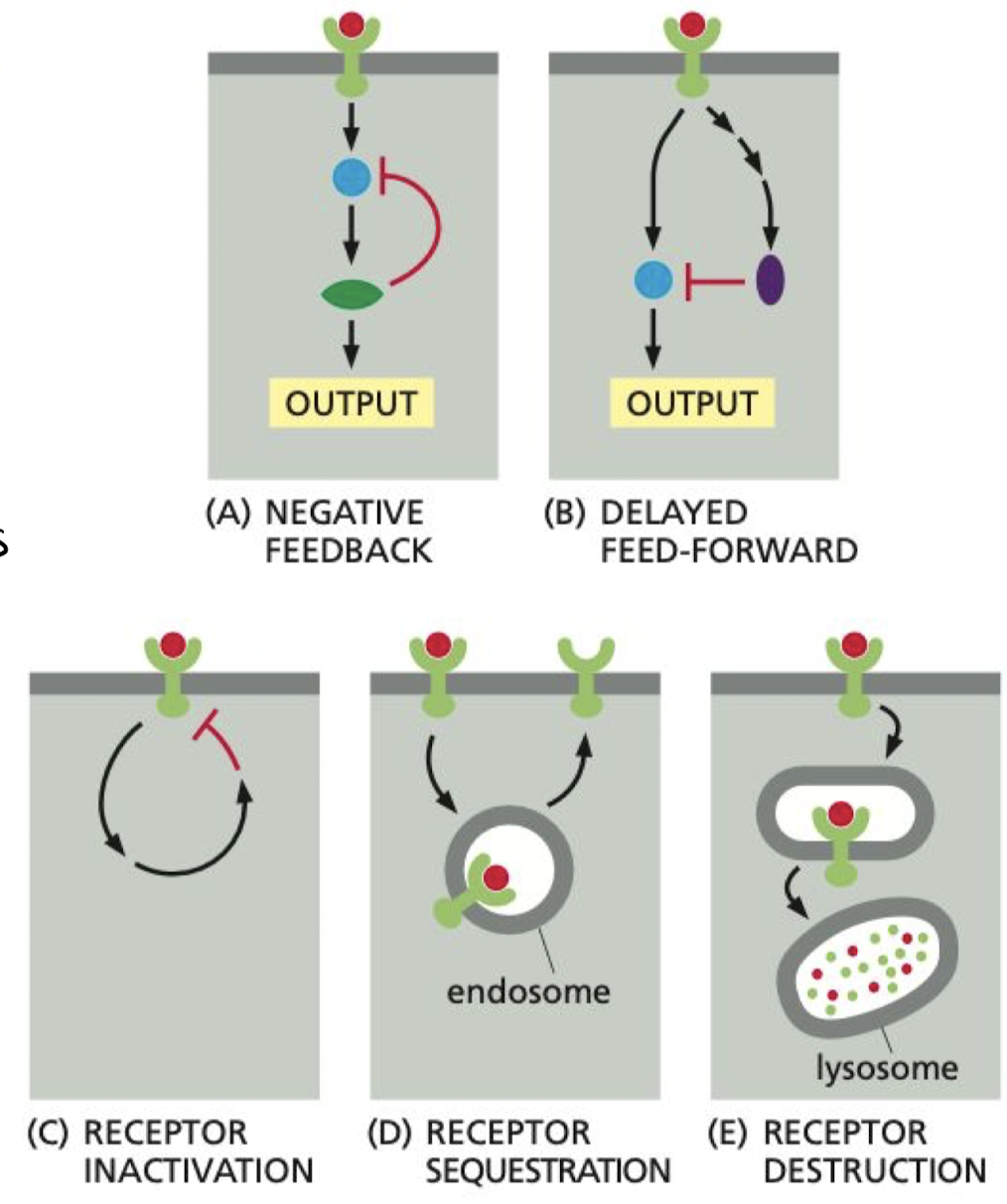
68
New cards
What are the steps in the relay of signals from GPCRs?
1. Binding of molecule to receptor causes release of GDP
2. GTP is allowed to bind to alpha subunit (Ga), activating it and the beta/gamma complex(Gby)
1. Ga and Gby move on to impact target proteins
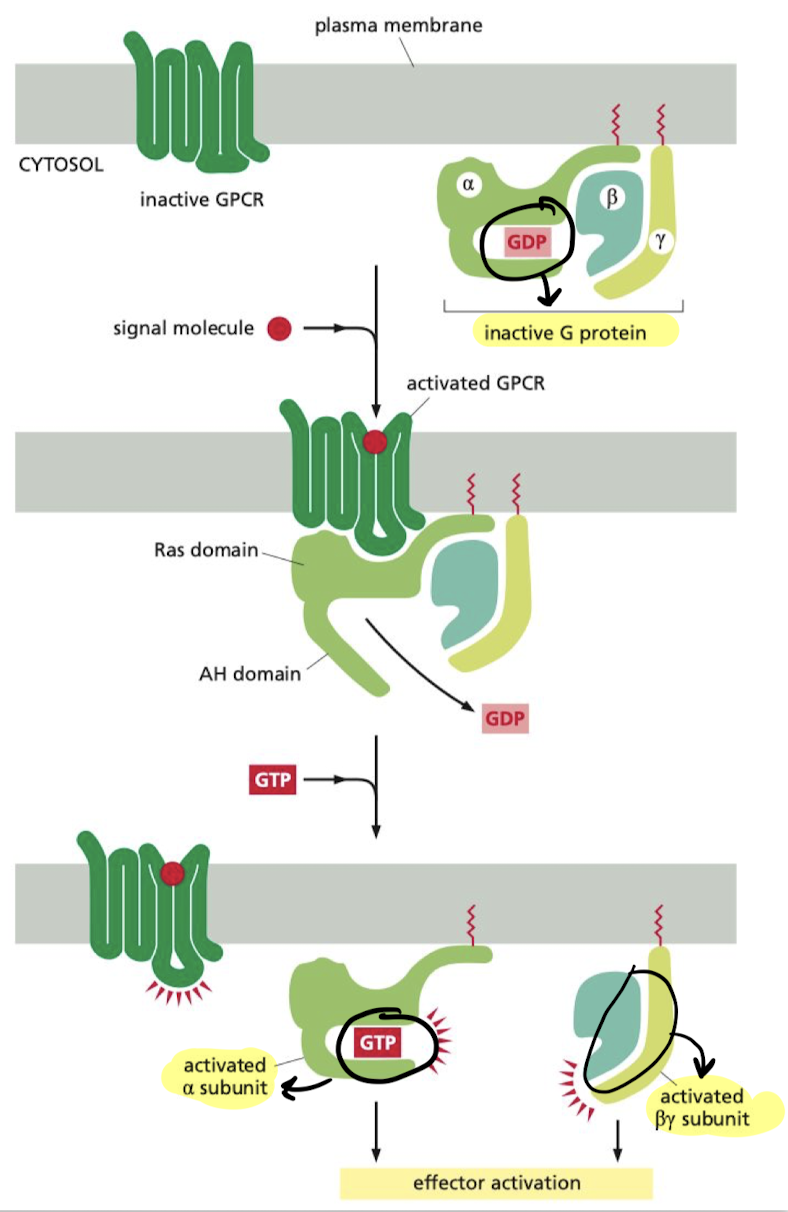
69
New cards
How is Cyclic AMP created, degredated, and reduced in the cell?
**Created** by adenylyl cyclase
**Degraded** by cyclic AMP phosphodiesterases
**Reduced** in the cell by inhibitory G proteins
**Degraded** by cyclic AMP phosphodiesterases
**Reduced** in the cell by inhibitory G proteins
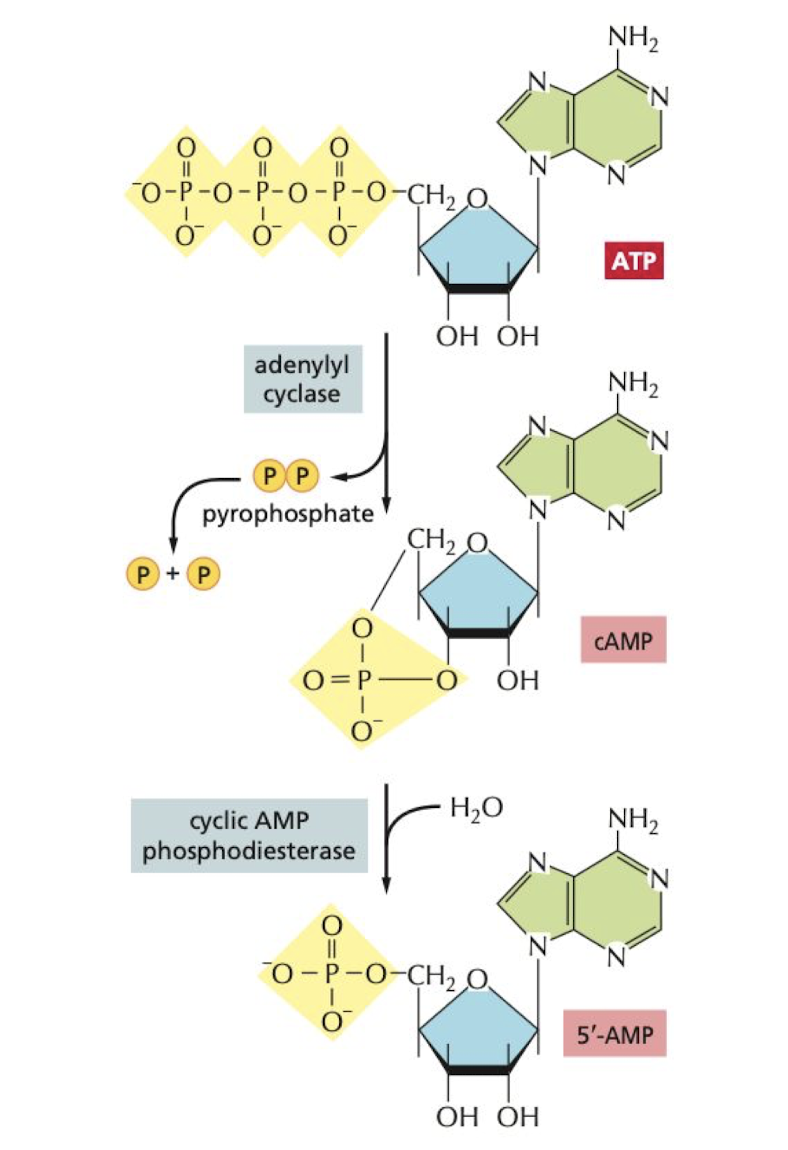
70
New cards
What is the main way that cAMP act in the cell?
activating cyclic-AMP-dependent protein kinase (protein kinase A; PKA).
* PKA target proteins differs from cell to cell, giving it a wide impact
* binding of cAMP to PKA activates kinase activity of catalytic subunits
* PKA target proteins differs from cell to cell, giving it a wide impact
* binding of cAMP to PKA activates kinase activity of catalytic subunits
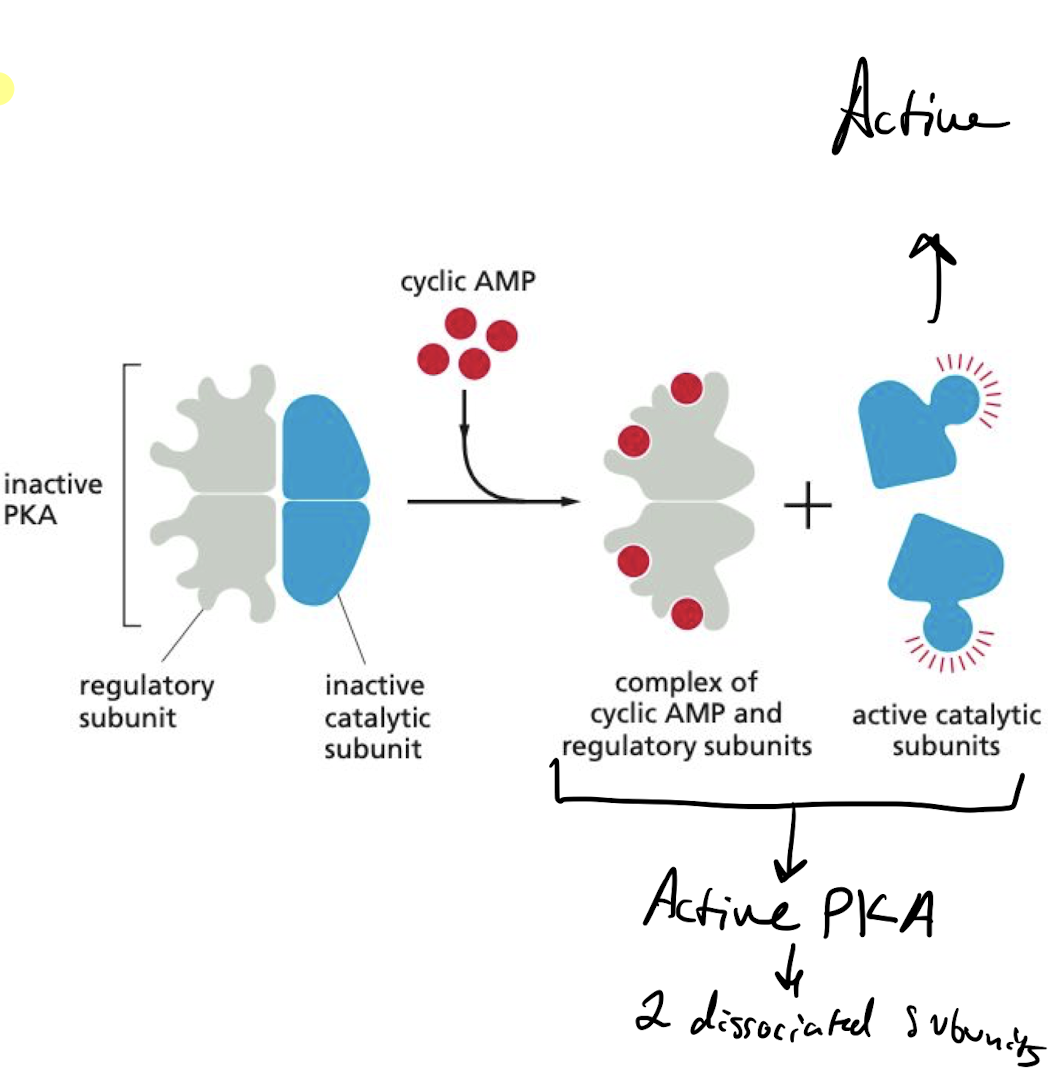
71
New cards
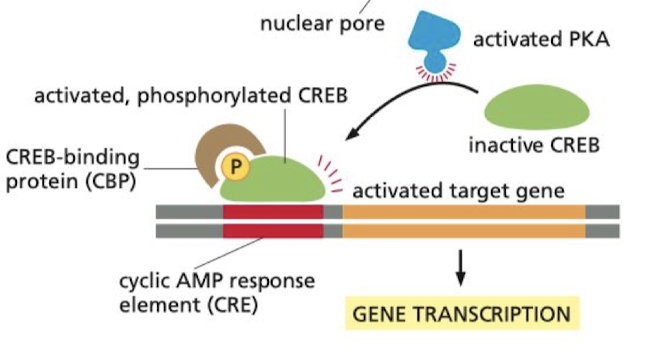
After being bound to cAMP, what is the effect of activated PKA on gene expression?
enters the nucleus and phosphorylates the transcription regulation protein CREB
* once phosphorylated, CREB recruits coactivator CBP to the CRE on the DNA, stimulating gene expression
* once phosphorylated, CREB recruits coactivator CBP to the CRE on the DNA, stimulating gene expression
72
New cards
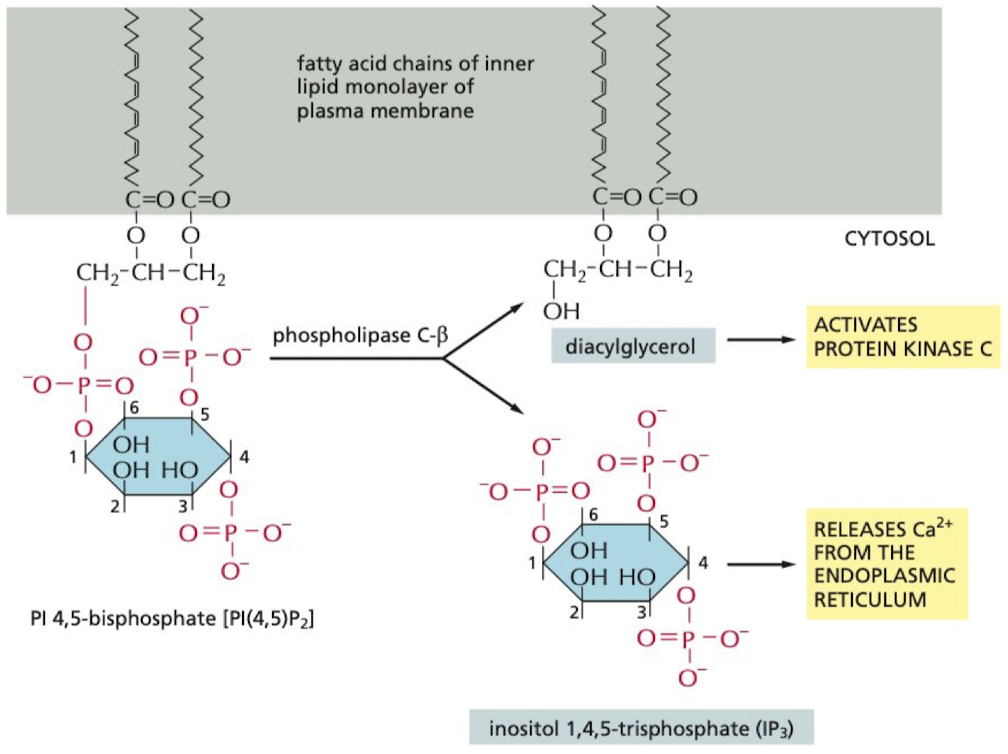
How do some G proteins signal via phospholipids?
hydrolysis of PI(4,5)P2 forms 2 secondary messengers
1. IP3 → releases Ca2+ from the ER
2. diacylglycerol → helps activate protein kinase C
1. IP3 → releases Ca2+ from the ER
2. diacylglycerol → helps activate protein kinase C
73
New cards
What cell signaling occurs when an egg is fertilized by sperm?
Ca2+ wave changes the egg cell surface, preventing the entry of other sperm
74
New cards
How are GPCRs used to facilitate our sense of smell?
when smell molecule binds to receptor, receptor acts to increase cAMP
* this activates an olfactory-specific G protein (Golf) → activates adenyl cyclase to increase cAMP
* this increase in cAMP opens cAMP-gated cation channels allowing influx of Na+ to send signal via AP
* this activates an olfactory-specific G protein (Golf) → activates adenyl cyclase to increase cAMP
* this increase in cAMP opens cAMP-gated cation channels allowing influx of Na+ to send signal via AP
75
New cards
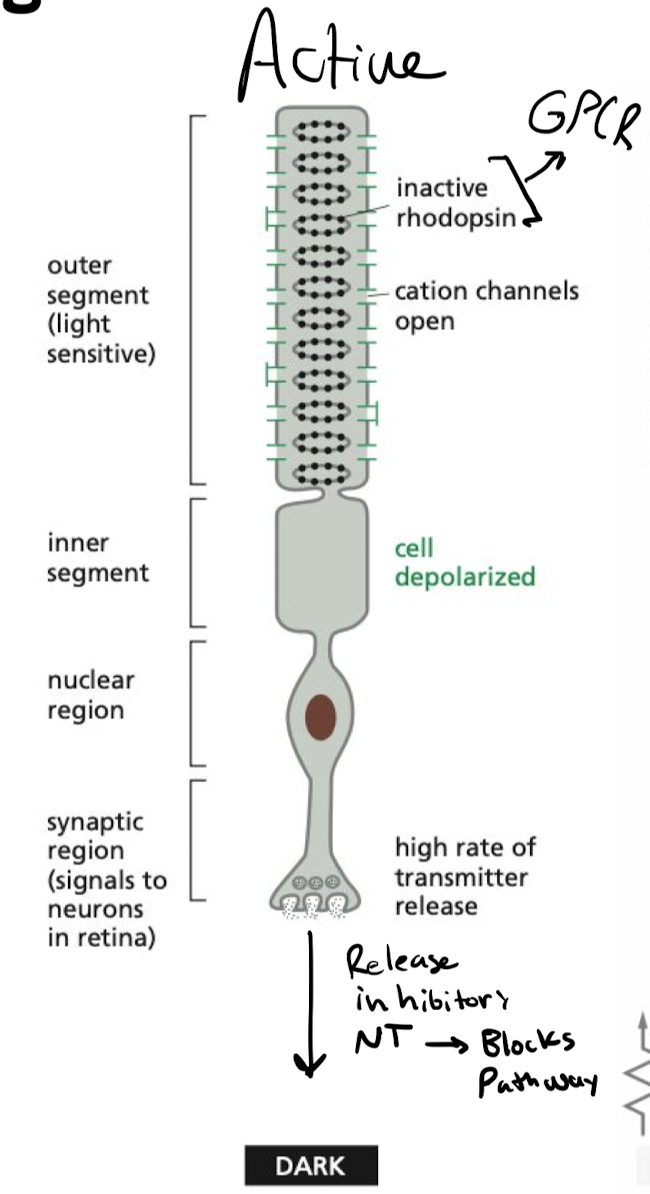
What keeps rod cells active in the absence of light?
Cyclic GMP is bound to cyclic GMP-gated cation channels, keeping them open in the dark, continuing the release of inhibitory NT
76
New cards
what inactivates rod cells in the presence of light?
presence of light activates rhodopsin in rod cell
* hyperpolarization inhibits synaptic signaling
* inhibitory signal stops, allows for rod cells to stimulate neurons
* hyperpolarization inhibits synaptic signaling
* inhibitory signal stops, allows for rod cells to stimulate neurons
77
New cards
A cell expresses a transmembrane protein that is cleaved at the plasma membrane to release an extracellular fragment. The fragment binds to receptor proteins on nearby cells and activates signaling pathways resulting in altered gene expression patterns in the cells. What form of intercellular signaling does this represent?
a. Contact-dependent signaling
b. Paracrine signaling
c. Synaptic signaling
d. Endocrine signaling
e. Autocrine signaling
a. Contact-dependent signaling
b. Paracrine signaling
c. Synaptic signaling
d. Endocrine signaling
e. Autocrine signaling
b. Paracrine signaling
78
New cards
Which of the following is NOT a common second messenger in cell signaling?
a. Ca2+
b. Cyclic adenosine monophosphate
c. Diacylglycerol
d. Tyrosine
e. Inositol trisphosphate
a. Ca2+
b. Cyclic adenosine monophosphate
c. Diacylglycerol
d. Tyrosine
e. Inositol trisphosphate
d. Tyrosine
79
New cards
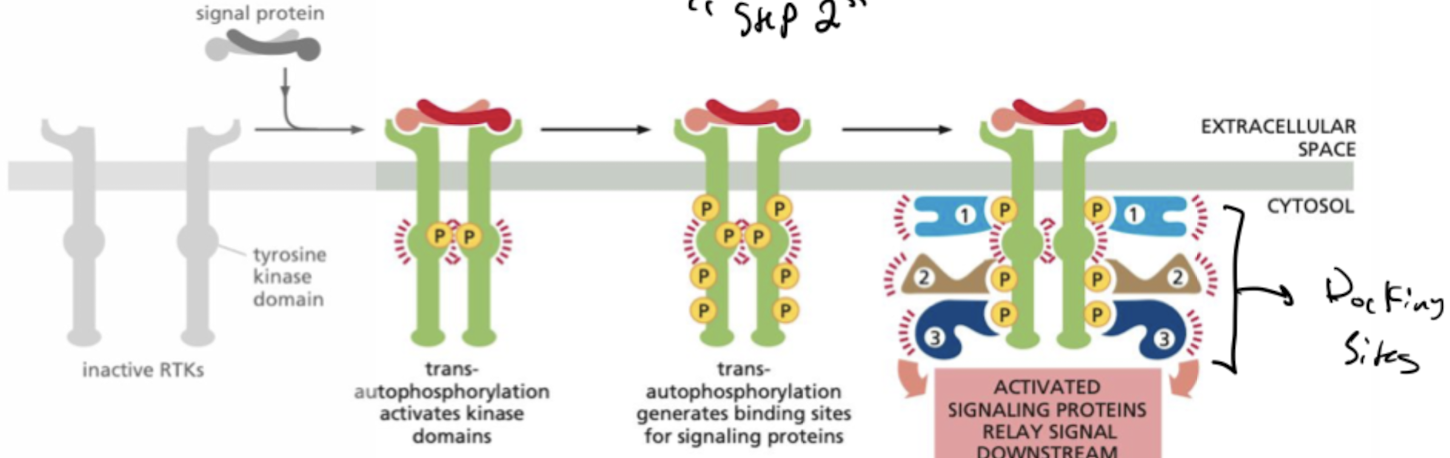
What are RTKs?
Receptor Tyrosine Kinases
* single pass proteins that are activated when bound to ligand phosphorylate themselves to allow binding of signal proteins
* single pass proteins that are activated when bound to ligand phosphorylate themselves to allow binding of signal proteins
80
New cards
What is Ras?
GTPase that relays cell-surface receptor signals
* often required for cell proliferation → mutated in cancers
* anchored to membrane via lipids
* often required for cell proliferation → mutated in cancers
* anchored to membrane via lipids
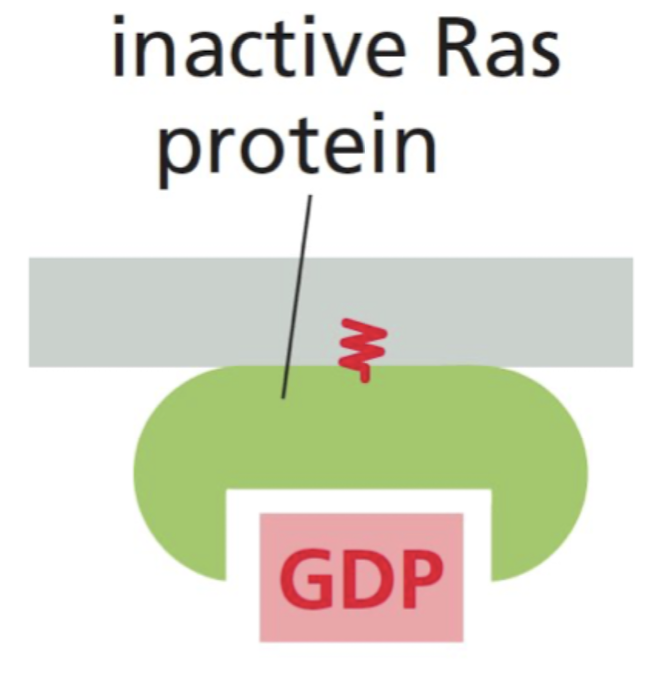
81
New cards
What is the function of GTPase-activating proteins(GAPs)
Turns GTPase “off” by increasing the rate of GTP hydrolysis
* some cancer cells with Ras mutations are resistant to GAPs and are locked into a GTP-bound state
* some cancer cells with Ras mutations are resistant to GAPs and are locked into a GTP-bound state
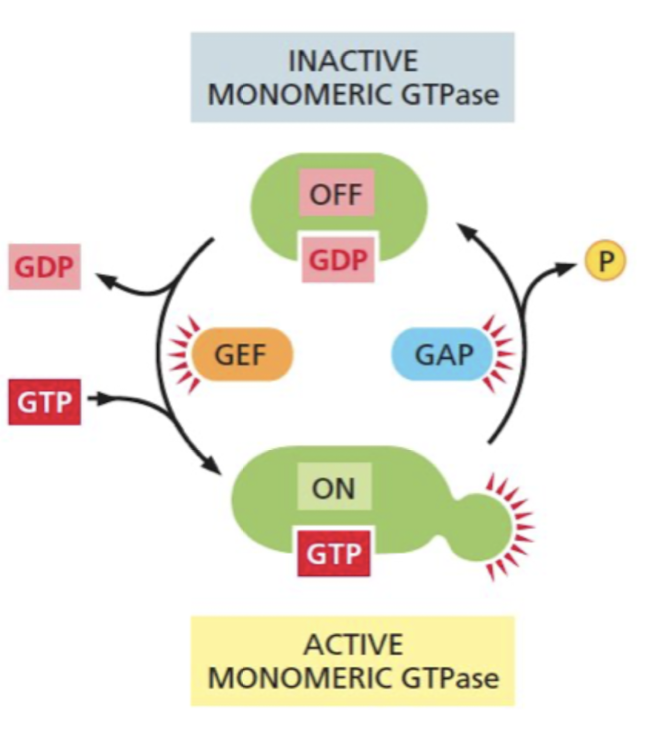
82
New cards
What is the function of guanine nucleotide exchange factors? (GEFs)
Turns GTPase “on” by promoting release of bound GDP, allowing more GTP to bind
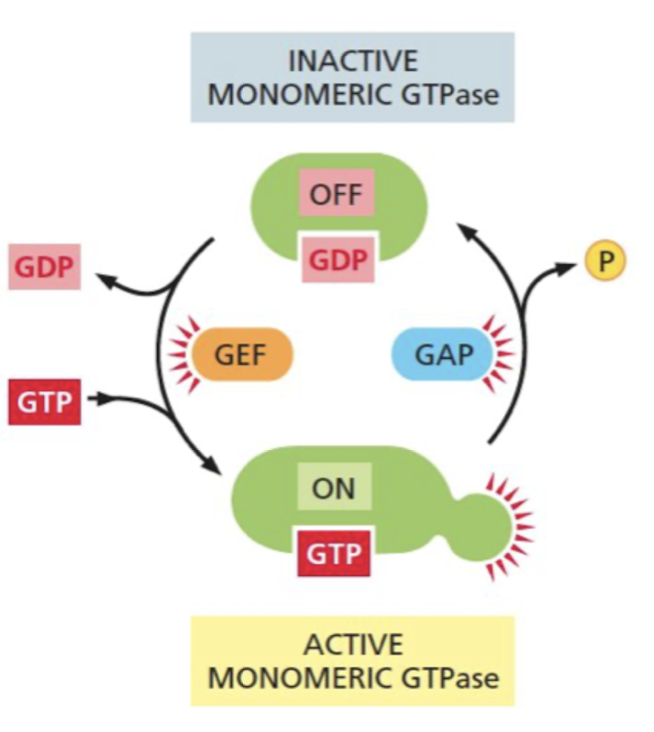
83
New cards
What are the steps in RTK passing signals to Ras GTPase?
1. SH2 domain proteins bind to phosphorylated tyrosines
2. Grb2 adaptor protein recognizes a specific phosphorylated tyrosine on the activated receptor via SH2 domain.
3. Grb2 recruits the Ras GEF, Sos
4. Ras GEF domain of Sos stimulates the inactive Ras protein to replace its bound GDP by GTP, which activates Ras to relay the signal downstream
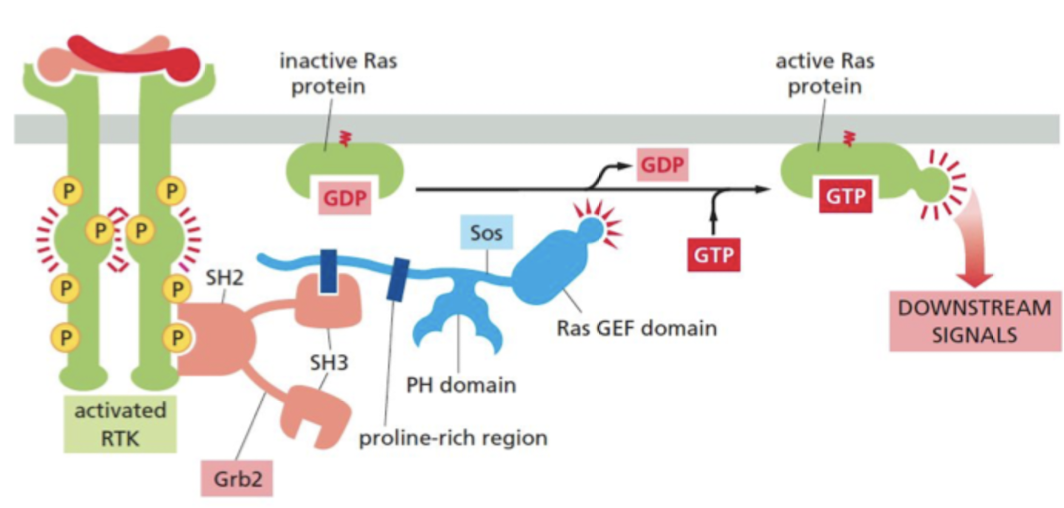
84
New cards
How does Ras GTPase transmit a signal to target proteins?
Ras activation causes kinase cascade
1. Ras recruits Ref
2. Ref recruits Mek
3. Mek recruits Erk
4. signal reaches target proteins
Protein response is either fast (changing protein shape) or slow (changing gene expression)
1. Ras recruits Ref
2. Ref recruits Mek
3. Mek recruits Erk
4. signal reaches target proteins
Protein response is either fast (changing protein shape) or slow (changing gene expression)
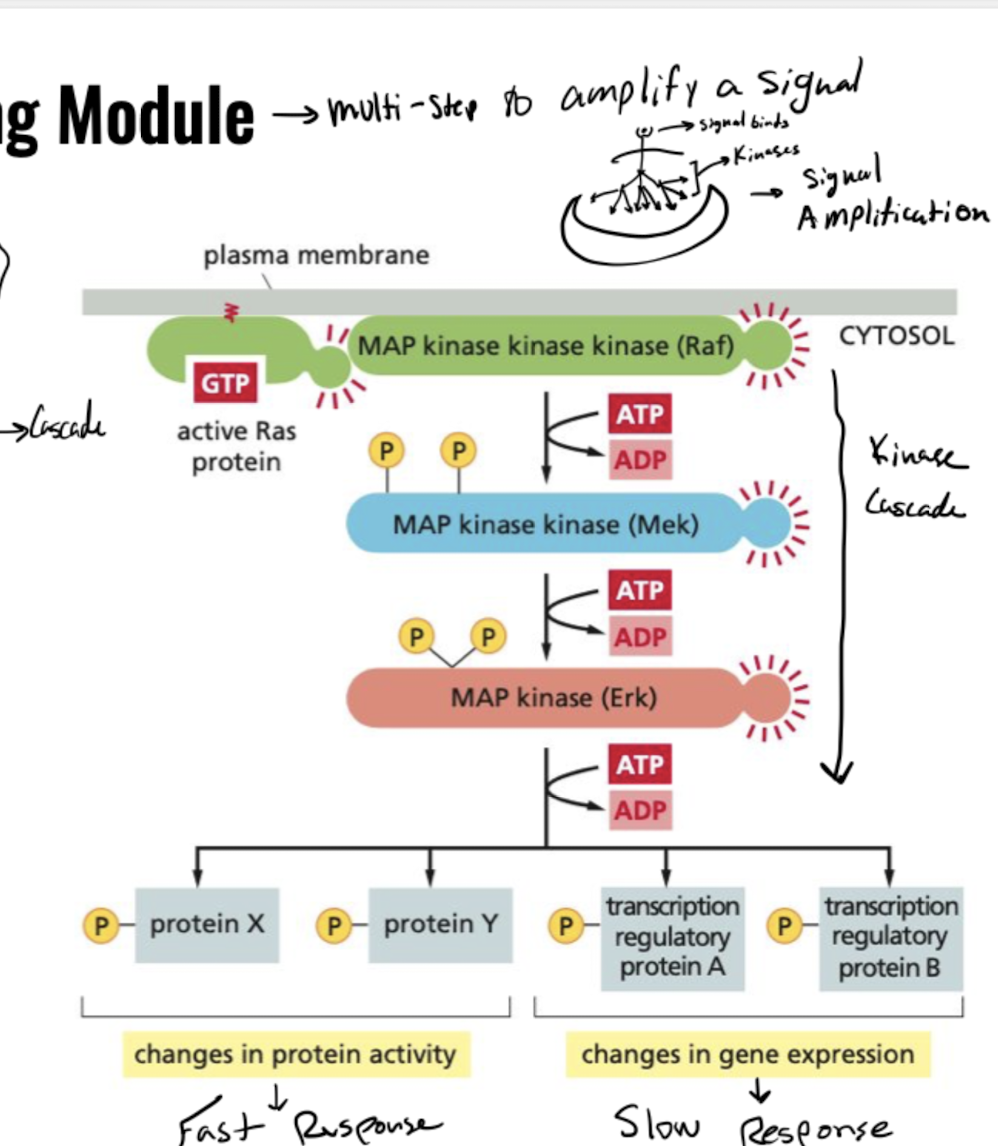
85
New cards
Besides proliferation, what other signaling pathway are RTKs used?
Cell survival pathway
* PI(3,4,5)P3 recruits Akt and phosphoinositide-dependent protein kinase 1 (PDK1) to the plasma membrane.
* Akt becomes activated, phosphorylates various target proteins at the plasma membrane, as well as in the cytosol and nucleus.
* Actions of Akt all work to enhance cell survival and growth.
* PI(3,4,5)P3 recruits Akt and phosphoinositide-dependent protein kinase 1 (PDK1) to the plasma membrane.
* Akt becomes activated, phosphorylates various target proteins at the plasma membrane, as well as in the cytosol and nucleus.
* Actions of Akt all work to enhance cell survival and growth.
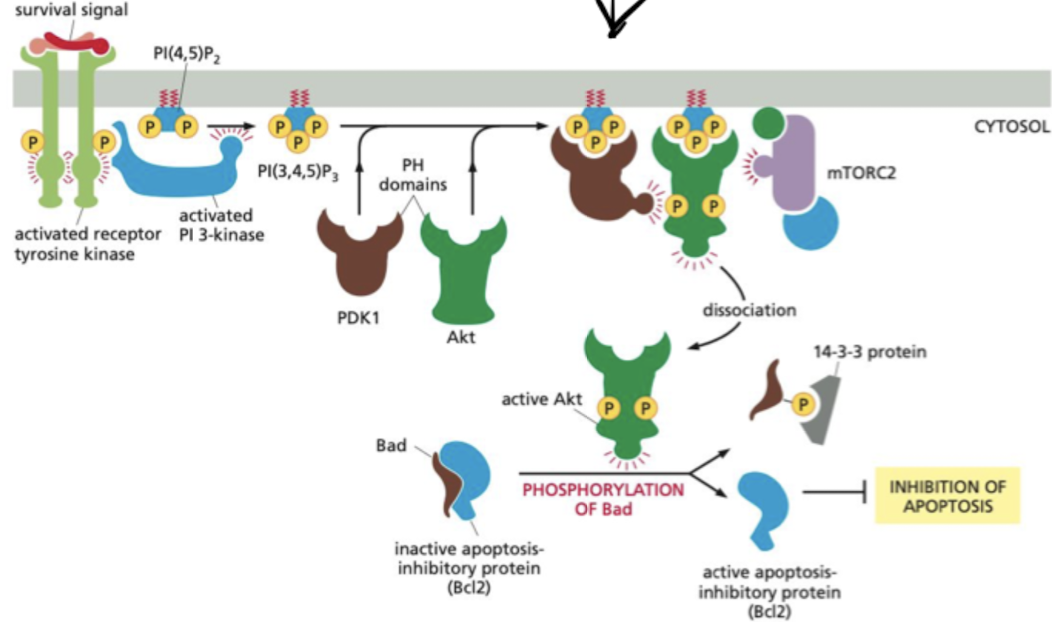
86
New cards
What kinase helps control the cell survival and growth pathway?
TOR
* mTORC1 → stimulates cell growth
* mTORC2 → activates Akt to promote cell survival
* mTORC1 → stimulates cell growth
* mTORC2 → activates Akt to promote cell survival
87
New cards
What do JAKs do?
JAK = Janus Kinases
* phosphorylate and activate transcription regulators called STATs
* phosphorylate and activate transcription regulators called STATs
88
New cards
What do STATs do?
migrate into the nucleus after they are activated and regulate gene transcription.
89
New cards
What are the steps in the JAK-STAT signaling pathway?
1. 2 cytokine receptors come together, bind to cytokine and phosphorylate each other’s JAKs
2. SH2 domain on JAK phosphorylates cytokine receptor to create docking site for STAT1 and STAT2
3. Activated transcription regulatory complex formed by STAT1 and STAT2 enter nucleus to activate gene transcription
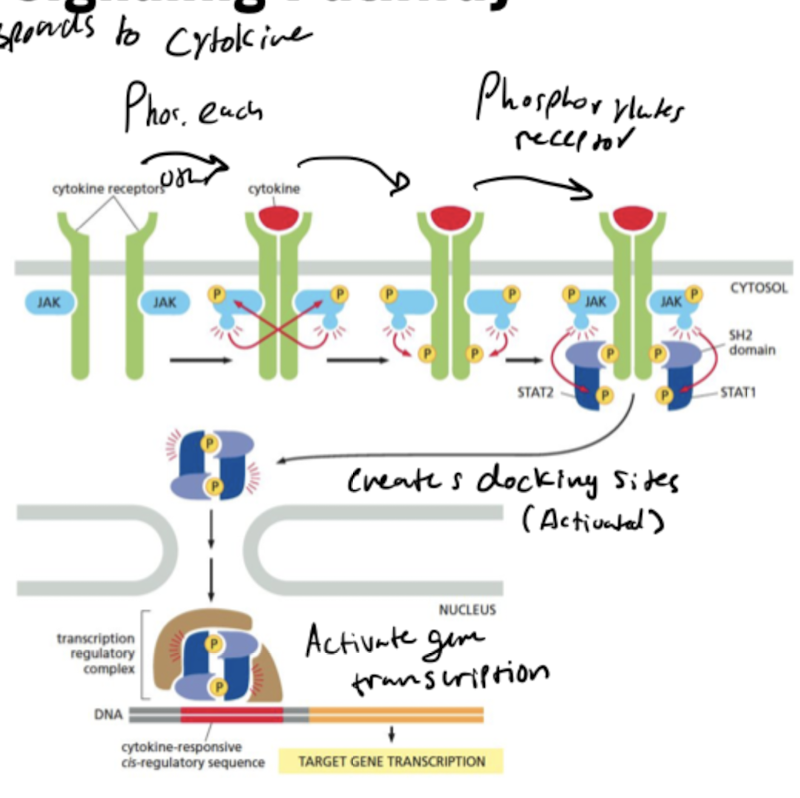
90
New cards
What does TGFb do?
regulates pattern formation and many other cell functions
91
New cards
What are the steps in the TGFb transcription pathway?
1. TGFβ dimer promotes assembly of a tetrameric receptor complex containing 2 copies each of the type-I and type-II receptors.
2. Type-II receptors phosphorylate specific sites on the type-I receptors, activating their kinase domains, leading to phosphorylation of R-Smads such as Smad2 and Smad3.
3. Smads open up to expose binding surface when phosphorylated, leading to the formation of a trimeric Smad complex containing two R-Smads and Smad4.
4. Phosphorylated Smad complex enters the nucleus and works with other regulators to control transcription of specific target genes.
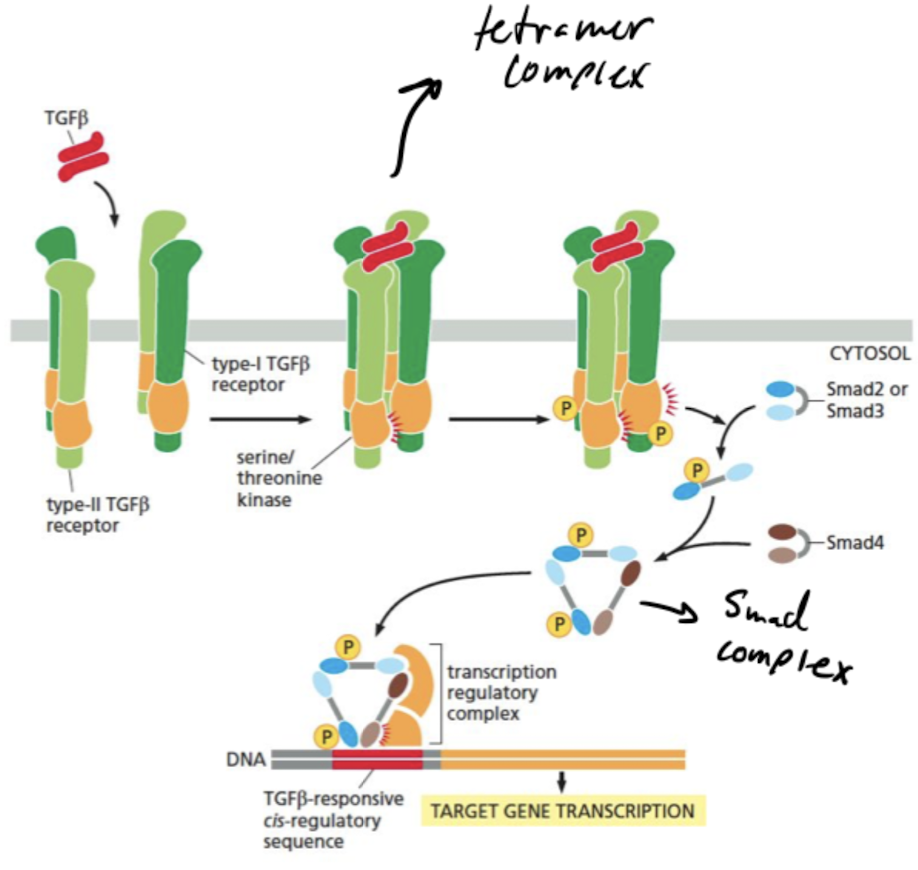
92
New cards
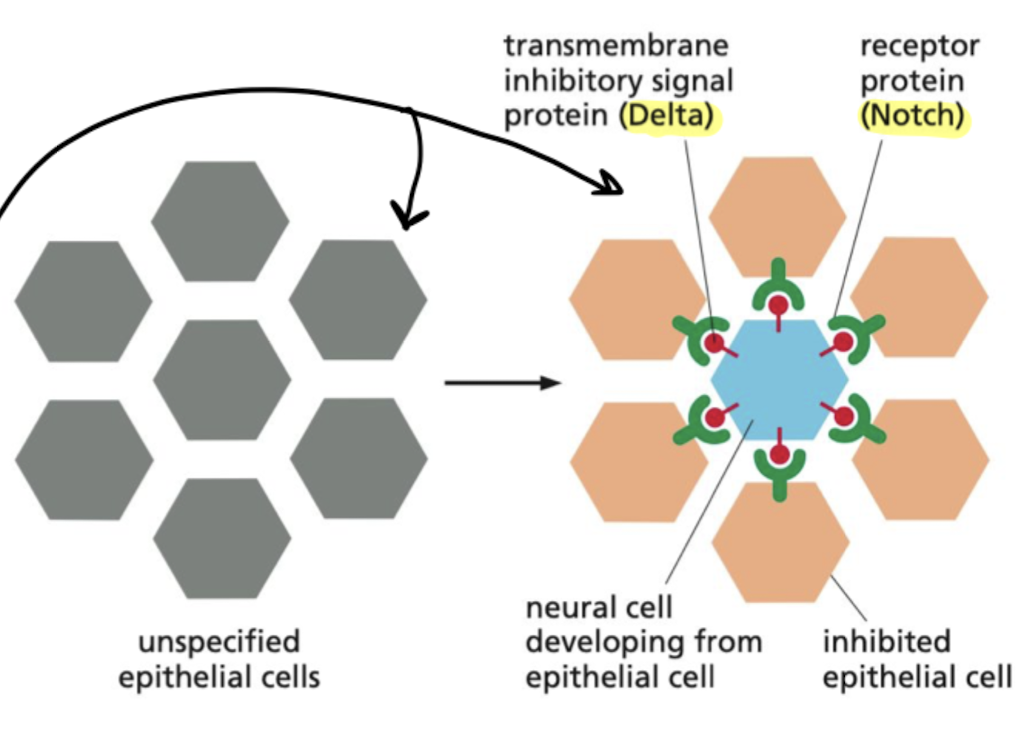
What is lateral inhibition?
When a precursor cell commits to becoming a neural cell, it signals to its immediate neighbors not to do the same; the inhibited cells develop into epidermal cells instead.
* depends on delta signal proteins and notch receptors
* occurs in development of fly neural cells
* depends on delta signal proteins and notch receptors
* occurs in development of fly neural cells
93
New cards
What is Notch and what are the steps for it acting as a latent transcription regulator?
single-pass transmembrane protein that requires proteolytic processing to function, acting as a latent transcription regulator
* activates by binding to delta, where its tail gets cleaved and translocates into nucleus to regulate transcription
* activates by binding to delta, where its tail gets cleaved and translocates into nucleus to regulate transcription
94
New cards
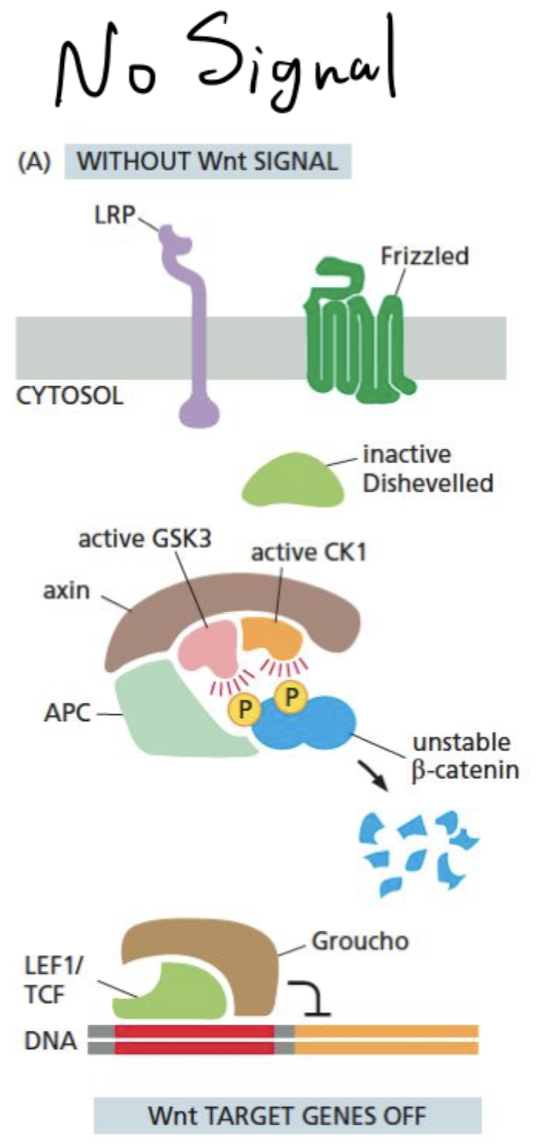
How does the Wnt pathway look in the absence of a signal?
* cytoplasmic beta-cantenin is degraded via phosphorylation from a degradation complex which also prevents it from binding to the nucleus
* Wnt-responsive genes in the nucleus are also suppressed by the Groucho co-repressor
\
* Wnt-responsive genes in the nucleus are also suppressed by the Groucho co-repressor
\
95
New cards
How does the Wnt pathway look when there is a signal?
* Wnt binds to Frizzled, LRP brings 2 co-receptors together
* CK1 and GSK3 phosphorylate LRP cystolic tail
* Activated Frizzled recruits Disheveled, disassembles degradation complex
* unphosphorylated β-catenin accumulates and translocates to the nucleus and binds to LEF1/TCF which displaces Groucho
* LEF1/TCF acts as coactivator to stimulate transcription of Wnt target genes
* CK1 and GSK3 phosphorylate LRP cystolic tail
* Activated Frizzled recruits Disheveled, disassembles degradation complex
* unphosphorylated β-catenin accumulates and translocates to the nucleus and binds to LEF1/TCF which displaces Groucho
* LEF1/TCF acts as coactivator to stimulate transcription of Wnt target genes
96
New cards
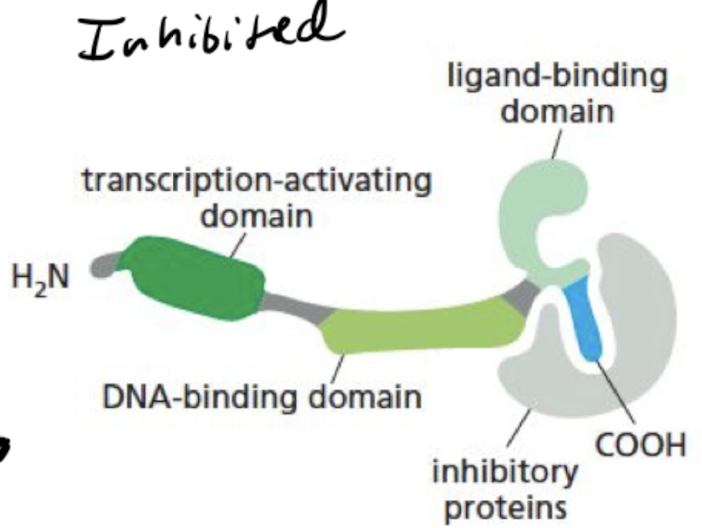
What does a nuclear receptor look like when its inactivated?
receptor is bound to inhibitory proteins
97
New cards
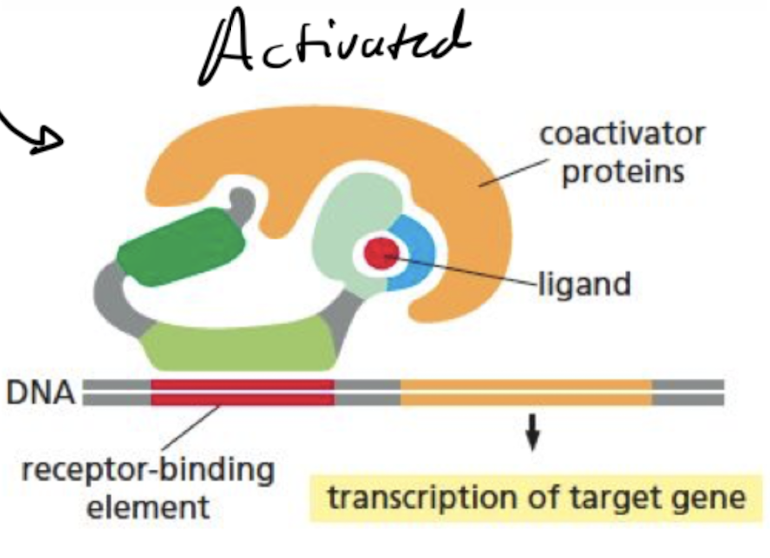
What does a nuclear receptor look like when its activated?
ligand-binding domain of the receptor clamps shut around the ligand, inhibitory proteins dissociate, and coactivator proteins bind to receptor’s transcription- activating domain, increasing gene transcription
98
New cards
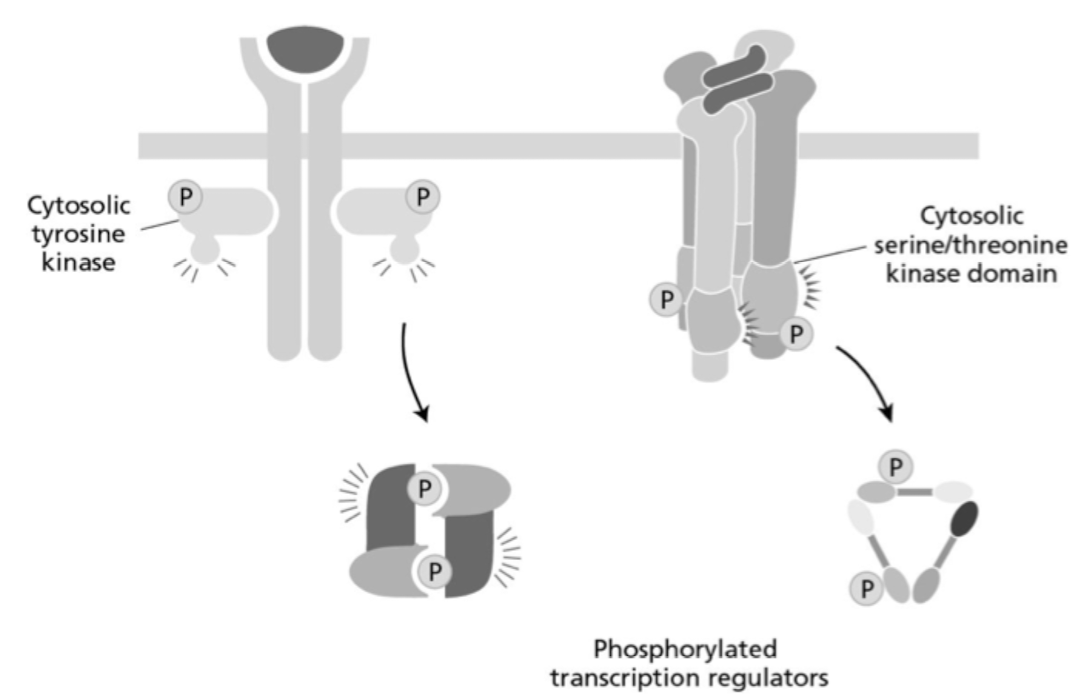
What two cell-surface receptors are represented in the two simplified diagrams below (from left to right)?
a. TGFβ receptor and TNF receptor
b. Cytokine receptor and TNF receptor
c. TNF receptor and TGFβ receptor
d. TGFβ receptor and cytokine receptor
e. Cytokine receptor and TGFβ receptor
a. TGFβ receptor and TNF receptor
b. Cytokine receptor and TNF receptor
c. TNF receptor and TGFβ receptor
d. TGFβ receptor and cytokine receptor
e. Cytokine receptor and TGFβ receptor
e. Cytokine receptor and TGFβ receptor
99
New cards
Signal transducers and activators of transcription (STATS) are latent transcription regulators, meaning they require activation in order to function. What is the name of the domain that is responsible for STAT function in the nucleus?
a. Ras
b. MAP
c. JAK
d. SH2
e. RTK
a. Ras
b. MAP
c. JAK
d. SH2
e. RTK
d. SH2
100
New cards
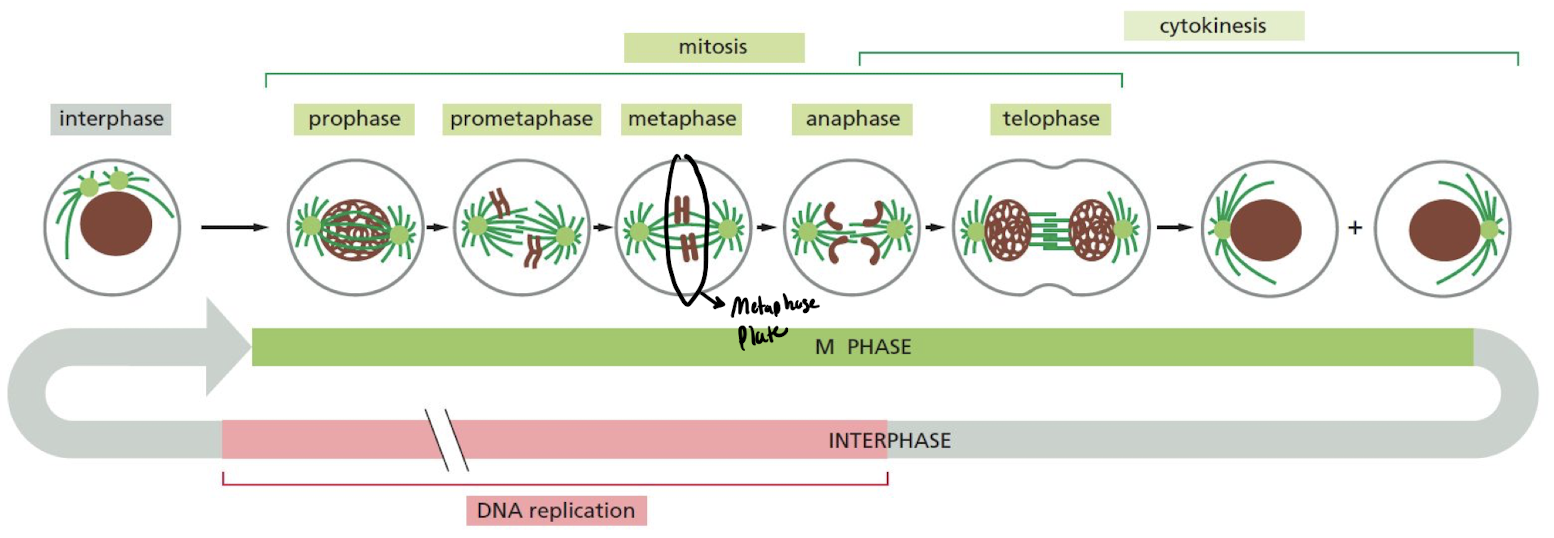
What are the 6 steps of cell division (M phase)?
1. **Prophase**: replicated chromosomes condense into sister chromatids
2. **Prometaphase**: nuclear envelope disassembles, sister-chromatid pairs attach to spindle fibers
3. **Metaphase**: sister chromatids align at metaphase plate
4. **Anaphase**: sister chromatids separate to either side of cell
5. **Telophase**: spindle disassemble + nuclear membrane assemble
6. **Cytokinesis**: cell divides