Stereochemistry and Haworth
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
How do we know if something is a D sugar?
if the furthest chiral centre from the carbonyl has a hydorxyl group on the right (dexter - right)
Anomeric Carbon
The carbon that was originally the carbonyl carbon in the linear form of a sugar; it becomes the new chiral center in the ring (C1 in aldoses, C2 in ketoses).
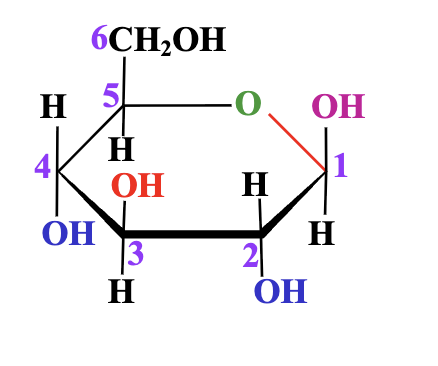
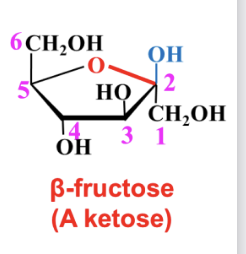
Haworth vs fischer projection
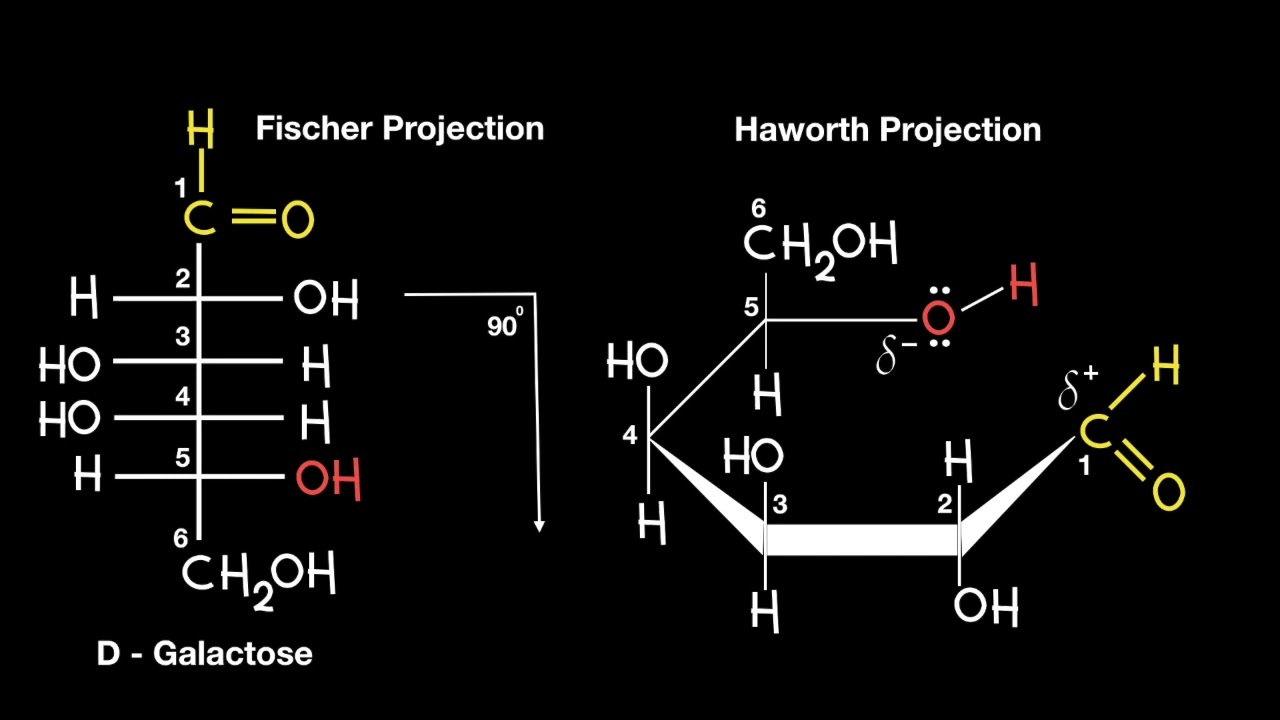
In a D-sugar, how do OH group positions in a Fischer projection relate to a Haworth projection?
OH groups on the left in Fischer are above the ring in the Haworth projection.

In a D-sugar, where is the OH group on the anomeric carbon in the β-anomer?
Above the ring.

In a D-sugar, where is the OH group on the anomeric carbon in the α-anomer?
Below the ring

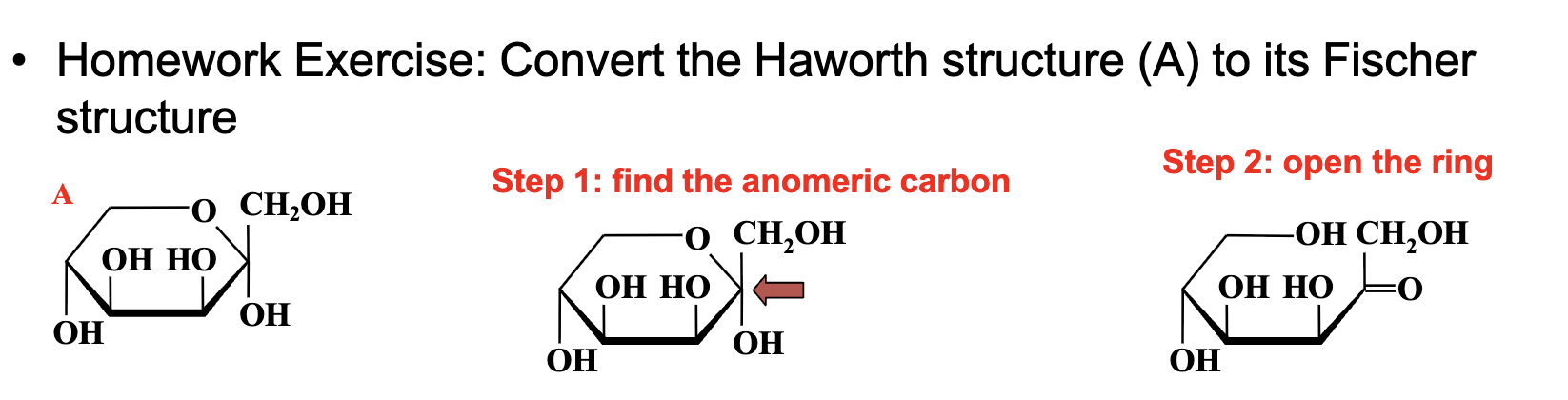
Instructions: Converting Fischer to Haworth Projections
Identify the carbonyl carbon (C=O) in the Fischer structure. This becomes the anomeric carbon in the ring.
Decide which OH group attacks the carbonyl carbon to form the ring:
For a pyranose ring (6-membered), the OH on C5 or C6 attacks.
For a furanose ring (5-membered), an OH closer to the carbonyl attacks (e.g., C4).
Form the ring: The oxygen from the attacking OH forms a bridge to the anomeric carbon, creating a cyclic hemiacetal (or hemiketal) structure.
Draw the ring with the oxygen at the back (upper right) in Haworth projection.
Assign each OH group as above or below the ring:
If the OH is on the right in the Fischer projection, it goes below the ring in Haworth.
If the OH is on the left in Fischer, it goes above the ring in Haworth.
Assign the anomeric OH (C1 for aldoses, C2 for ketoses):
OH above the ring = β (beta) form
OH below the ring = α (alpha) form
Add any substituents (e.g., CH2OH) as appropriate (usually above the ring for D-sugars).
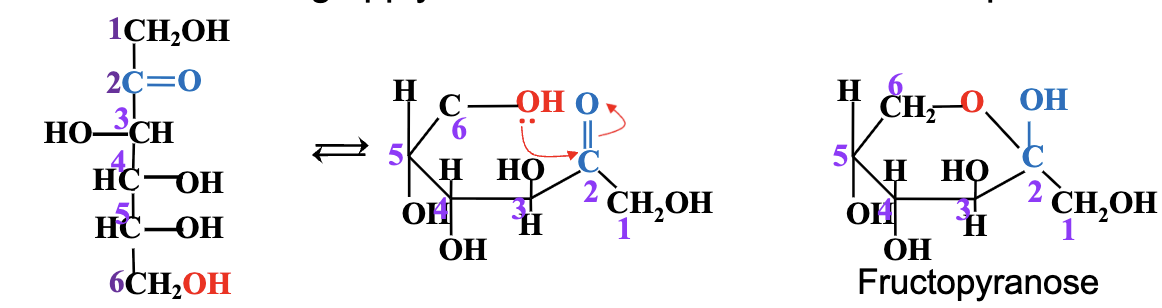
Additional notes:
The size of the ring depends on which OH attacks — smaller rings (furanose) or larger rings (pyranose).
In fructose, if the OH on C6 attacks the carbonyl carbon, a pyranose ring forms; if OH on C5 attacks, a furanose ring forms.

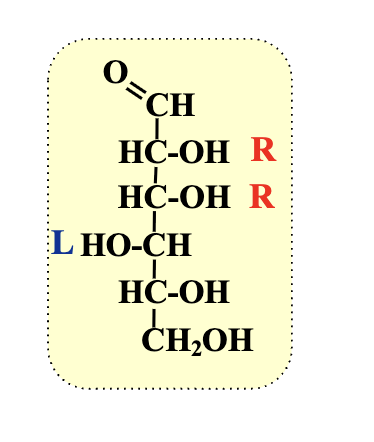
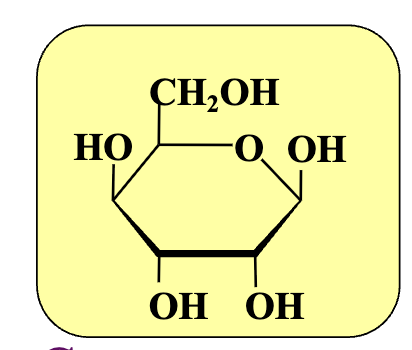
What carbon becomes the anomeric carbon when converting Fischer to Haworth?
The carbonyl carbon in the Fischer projection.
For D-sugars, where is the CH2OH group usually drawn in Haworth?
Above the ring.
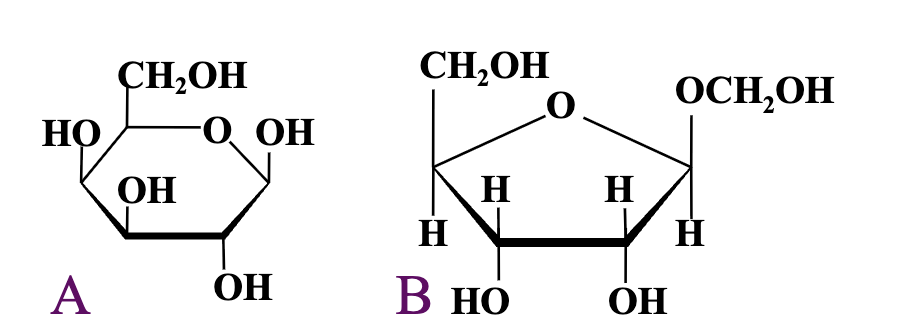
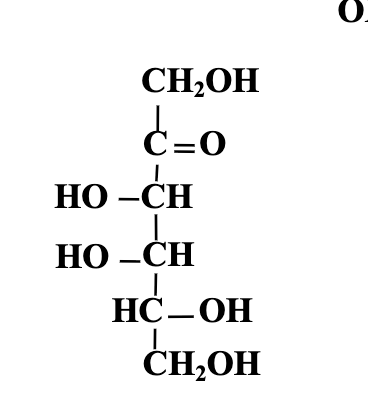
keep in mind left above, beta above
6 member ring attack from which carbon?
5 member ring attach from ehich carbon
OH on C5 attacks carbonyl carbon
OH on carbon 4 attacks carbonyl carbon to form 5 member ring