Psych 439 Final; synapse formation
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Prokaryotes; precursors to synapses
no need for cell-cell signaling (single cell) but need to respond to environmental signals
Therefore, they have sensors, scaffolding proteins,ion channels and receptors that can change gene transcription (similar to a neuron)
What are thre three components of a synapse?
presynaptic terminal
post synaptic region
synaptic cleft
presynaptic terminal
contains NT filled synaptic vesicles and machinary for release
post synaptic region
includes one or more postsynaptic densities (PSDs) that are opposite to the presynaptic terminal’s active zone and have receptors to receive signals
Synaptic cleft
area btwn two components filled w/extracellular matrix, compozed of a variety of long chain molecules that polymerize with one another (collagen, laminin, fibronectin)
What are the three stages of synaptic maturation?
initial contact
assembly of synaptic machinery
stabilization of synapse
What does a synapse begin with? What are the major components of this?
adhesion
NCAM, cadherins, beta catenin
NCAM
cell adhesion mol that helpd them grip surfaces (not specific)
Cadherins
homophilic specificity helps axons and targets display some discrimination and find the correct position
activation of each induces further discrimination
Beta-catenin
links intracellular domain of cadherin receptor to actin filaments inside cell to anchor synapse
cadherin-beta-catenin complex is important for pruning of dendritic spines
Beta-catenin is in short supply (only strongest spines get use, weak ones are pruned; ie, competition)
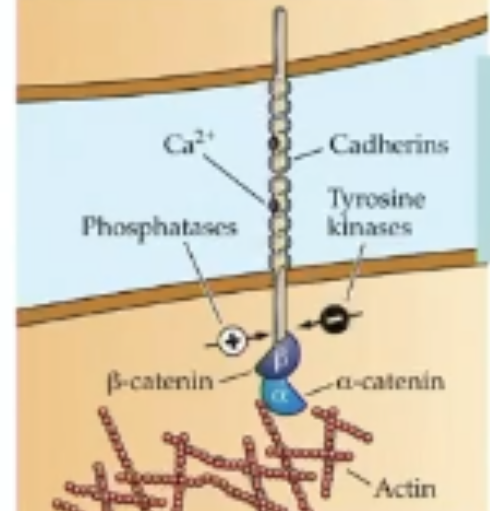
What type of binding does cadherins facilitate? What ion does it depend on?
homophilic binding
Ca2+
What factor is the density of synpatic spines in cortical neurons dependent on?
age!
childhhos: small synpatic spines
adolescense; huge increase in synaptic spines
adulthood (age 20); pruning results in selective existence of dendritic spines
What is Fragile X syndrome?
what occurs when weak/ineffective synapses aren’t pruned?
extended repeats of trinucleotide CGG leads to increased availability of scaffolding proteins
leading to overabundance of synapses (even weak ones) due to lack of competition
X-linked disorder; motor deficiencies, intellectual impairments, delayed onset of abilities
How does an axonal growth cone determine which targets are suitable?
detemined by adhesive molecules
What occurs after the axonal growth cone has contacted a suitable target?
axon and target induce each other to construct the apparatus needed for synaptic signaline
inductive factors; neurexin, neuroligin
Neurexin
inductive factor on incoming axon (NT exit)
Neuroligin
inductive factor on postsynaptic site (where ligand wld bind)
What occurs when neurexin and neuroligin binds?
induces machinery on both sides to form
assembly of postsynaptic density proteins (integral for scaffolding)
induces snare vesicles (snare complexes anchor vesicles to presynaptic membrane)
What is the NMJ?
neuromuscular junction
Development of muscle fibers
satellite cells—>dividing myoblasts—>initial myotube formation—>myotube maturation—>muscle fiber
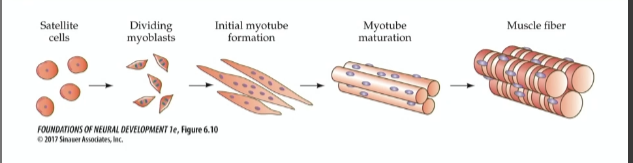
Myotubes
where actin and myosin organize
What facilitates maturation of myotubes into muscle fibers?
arrival of motor neuron axonal growth cone
components of NMJ
presynaptic axon terminal is encased by terminal schwann cell (peripheral) which isolates it from other influences
The muscle forms deep junctional folds rich is acetylcholinesterase
ACH receptors are concentrated at the tops of fold nearest axon terminal
basal lamina sheath (layers of ECM) btwn axon and muscle
How is the NMJ set up?
migrating growth cone constantly releases ACH as it gets toward end of journey, myotubes that express ACHRs
ACHR that are embedded in muscle cell migrate to location of developing synapse at incoming growth cone
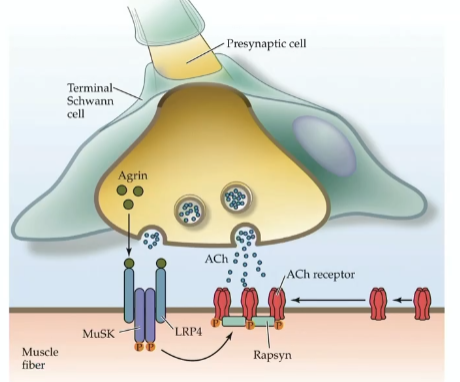
Agrin anchors the ACHRs so they are kept at the synapse rather than moving around in the lipid bilayer
Agrin
proteoglycan released from growth cone servin as signal and inductive factor at NMJ
How does agrin keep ACHR in place?
agrin binds to complex receptor on muscle cell (comprised of protein LPR4 and MuSK)
once bound, agrin activates complex—>internal domain of MuSK phosphorylates itself—>further clustering and activation of cytoplasmic protein rapsyn
rapsyn binds intracellular domain of several ACHRs and anchors them to microtubule of muscle cell
Once ACHRs are placed, new ones have to be made, how does that occur?
family of proteins called neuregulins induce muscles to increase trancrptions of various subunits of the ACHR at NMJ
How do neuregulins work?
embedded in motor neuronal axon membrane—>can act via cell to cell contact
can also be released (releasing factor but only locally)
binds to ErbB receptor tyrosine kinases on muscle fibers—>boost expression of ACHR subunits
What does the electrical activity of a cell depend on?
can change throughout development depending on ion channels
What are the two major types of ion channels?
voltage gated (Na, Ca2+, K+, Cl- channels); open due to voltage sensor detecting a change in charge
ligan gated: open in response to ligands like NTs, specific ions, or nucleotides
What changes in ions drive depolarization
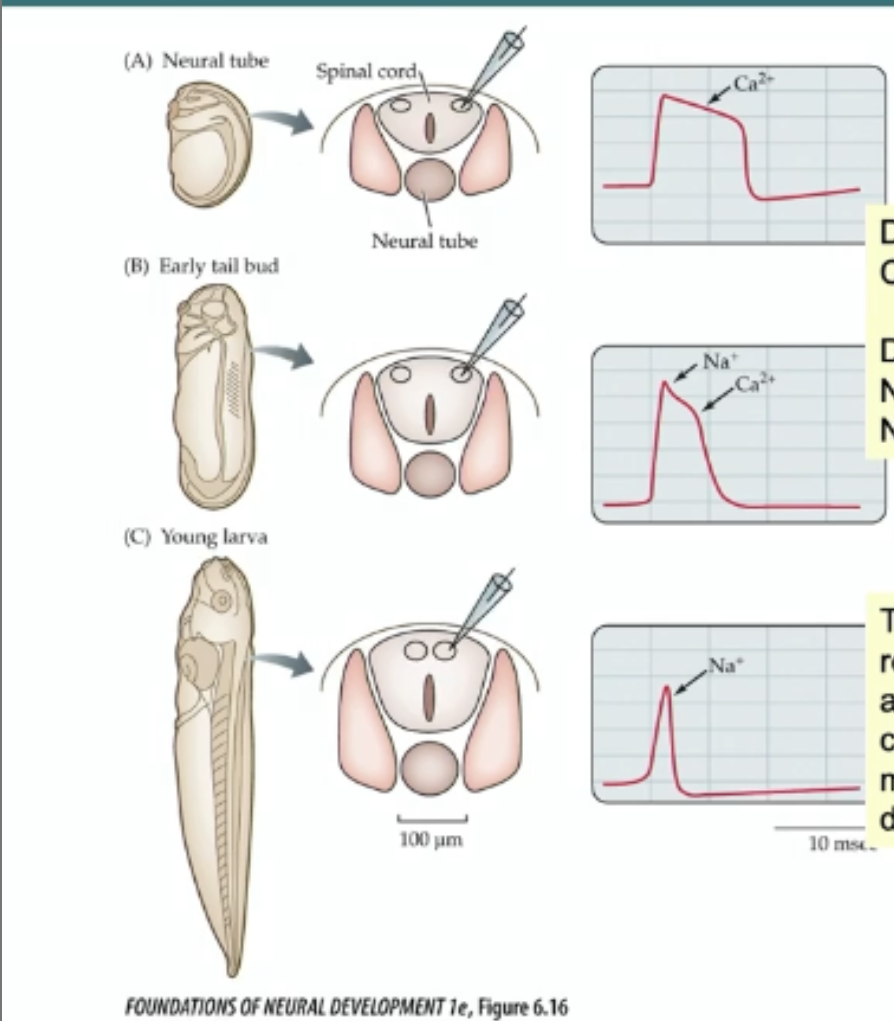
influx of Na+ (into cell via voltage gates Na+ channels) drives depolarization of a MATURE neuron
in embryonic neurons; influx of Ca2+ ions through voltage gated Ca2+ channels drive action potentials
Development of action potential
depolarization driven by Ca+ early on (slow recovery)
depolarization switches to NA+ as voltge gated An+ channels are added (faster recovery)
in young larva quicker recovery response is driven by addition of voltage gated K+ channels
How do ligand gated channels change across development?
function of ligand gated channels change across dev
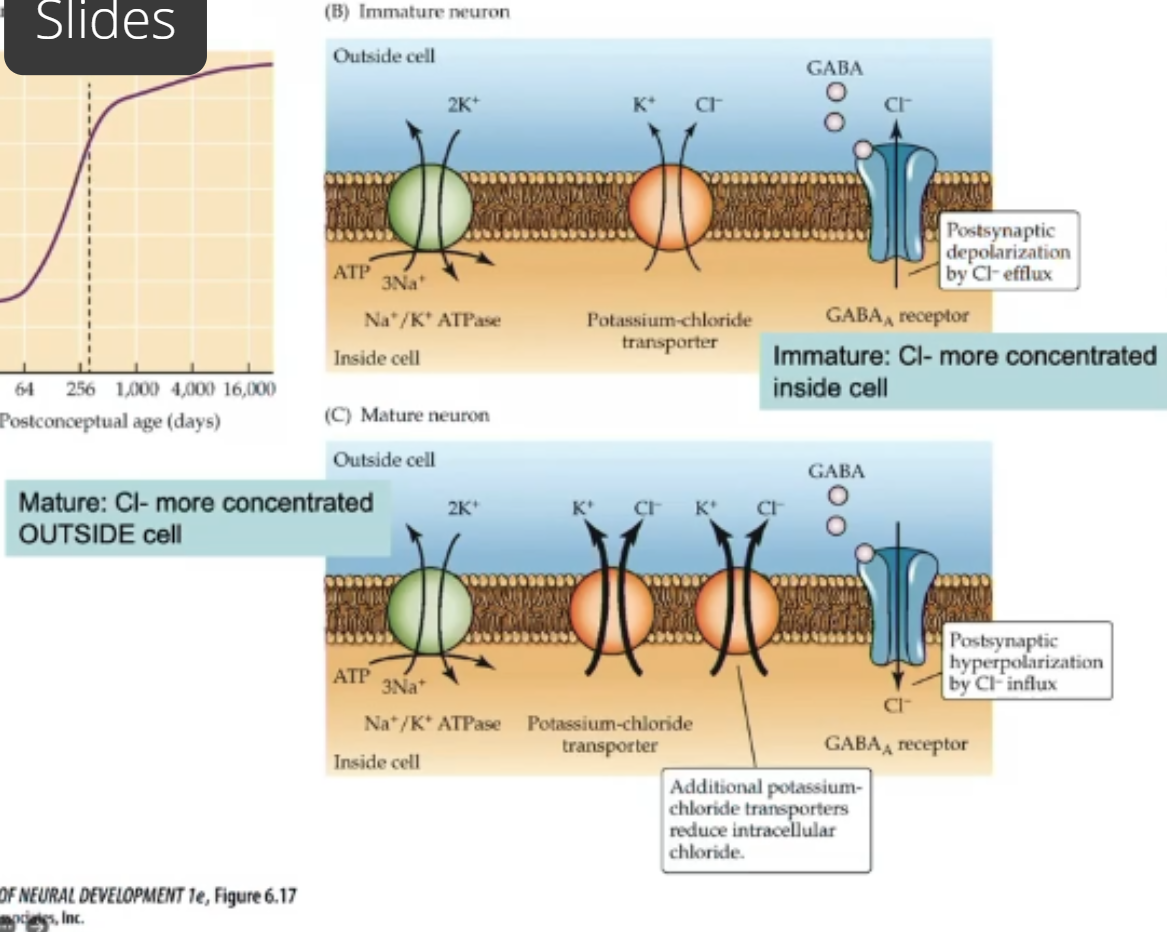
GABA receptors in mature NS are inhibitory (depolarization allows Cl- into cell)
in dev brain they’re excitatory; potassium chloride cotransporter not fully expressed; immature neurons have more chloride inside cell. When GABA receptor is activated Cl- exits cell(depolarization)
GABA binding to receptor in mature NS causes Cl- to enter cell—>hyperpolarize
Why does GABA switch from excitatory to inhibitory?
to ensure that the synapses are in appropriate spots, stengthened
Immature neuron: some K+Cl- cotransporters but not alot—>build up of Cl inside cell. When GABA binds Cl- moves out of cell (down concentration gradient)—>depolarization
Mature neurons: more K+CL- cotransporters—>more CL- outside cell. When GABA binds Cl- moves inside cell (down concentration gradient)—> hyperpolarization
How do ion channels change during dev?
ion channels change subunits (and therefor characteristics)
ion channels often made up of many subunits (derived from separate genes)
Expression of subunits change during dev—>electrical propertis of neurons also change
Embryonic synapses are slow, why?
Takes longer for presyaptic machinery to release NT—>longer delay btwn arrival of AP to axon terminal and peak of EPSP in postsynaptic cell
postsynaptic machinery also sluggish—> drawn out post synaptic potentials
ex. a cortical EPSP is 400 ms long in newborn rats and only 100 ms 2 weeks later (due to subunits that make up the postsynaptic receptor
Shortening of PSP is due to changes in function of NT receptor; stays open longer in young animals and faster rin adulthood (shortening of time due to change in subunits)