BIOCHEM TEST 1 (The Three Dimensional Structure of Proteins)
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
Q: What is the native state of a protein?
A: It's the most stable, functional 3D shape of a protein, where it has the lowest energy and maximum entropy.
Q: What's the difference between conformation and configuration?
A: Conformation is the shape due to rotation around single bonds; configuration is the fixed arrangement that can only change by breaking bonds.
Q: Why is the correct three-dimensional structure of a protein essential to its function?
A protein must fold into its native CONFORMATION to become biologically active. It allows it to interact with other molecules.
Q: Why is the amino acid sequence important in protein structure and function?
A: The sequence (primary structure) determines how the protein folds into its secondary, tertiary, and quaternary structures. Even one change in the sequence can disrupt folding and function
Q: What is protein breathing?
A: Small, constant fluctuations in a protein's shape as weak bonds break and reform.
Q: What are secondary (2°) structures in proteins? What bonds are involved?
A: Regular repeating structures in localized regions of the protein, stabilized by hydrogen bonds between the carbonyl oxygen and amido hydrogen of the peptide bond.
Q: What are the main types of secondary structures? (4)
Right handed α-helix
β-sheet (parallel and antiparallel)
Triple helix
turns (β-turns), and bends
What is an α-helix? What direction do the side chains project?
A right-handed spiral stabilized by H-bonds between carbonyl oxygen and amido hydrogen four residues ahead. Side chains project outward, perpendicular to the helix axis.
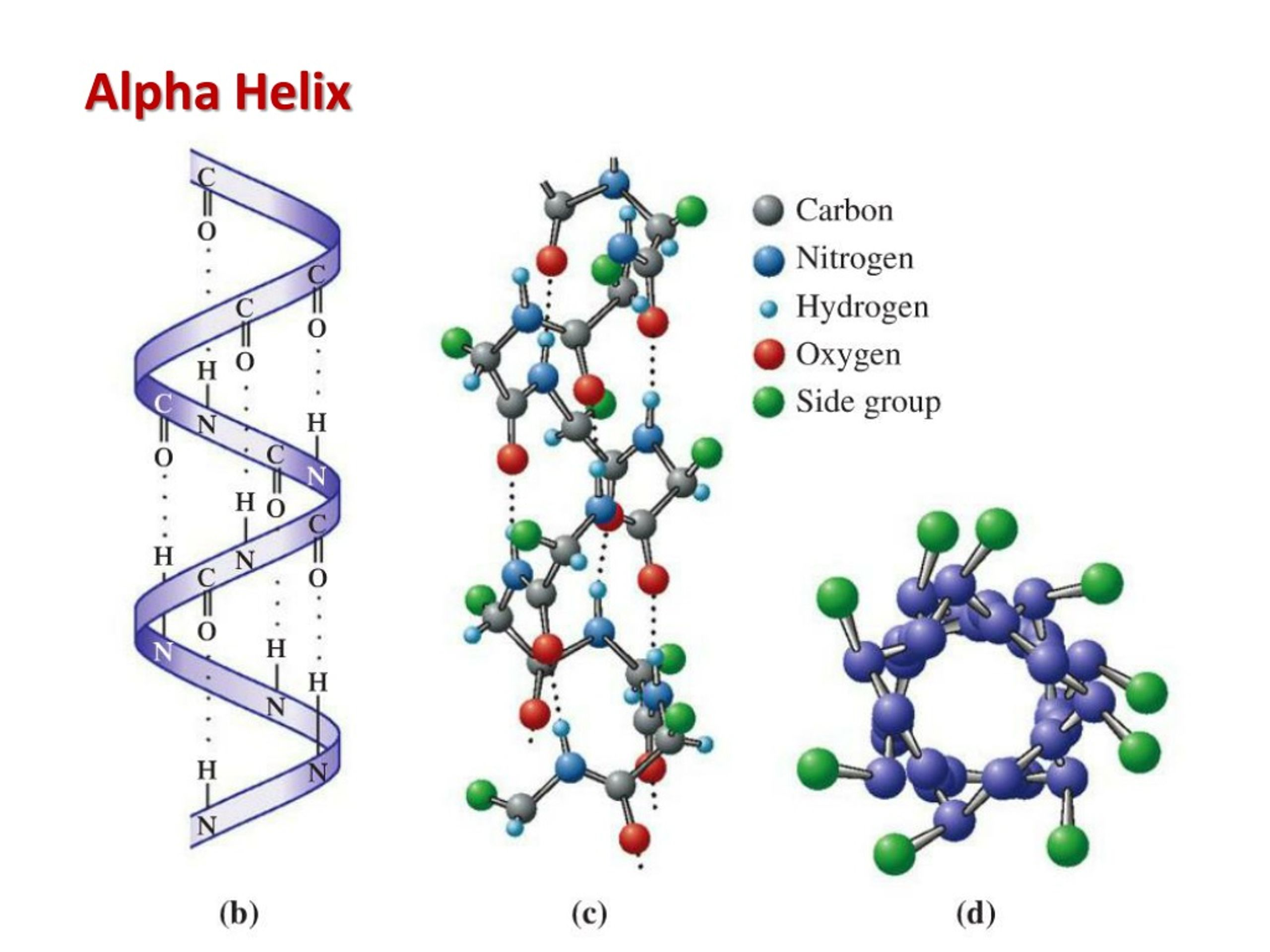
Q: What can disrupt α-helix formation? (3)
Proline (kinks the helix)
glycine (too flexible)
adjacent bulky or like-charged side chains (steric/electrostatic hindrance).
Q: What is helix capping?
A: The folding of polypeptide chain to provide H-bond partners for the unpaired groups at the helix ends, stabilizing the helix.
Q: What is a β-sheet? What bonds stabilize them?
A: A more extended structure with strands running parallel or antiparallel, stabilized by H-bonds between neighboring strands.
Q: How are R groups arranged in β-sheets?
A: They alternate above and below the plane of the sheet.
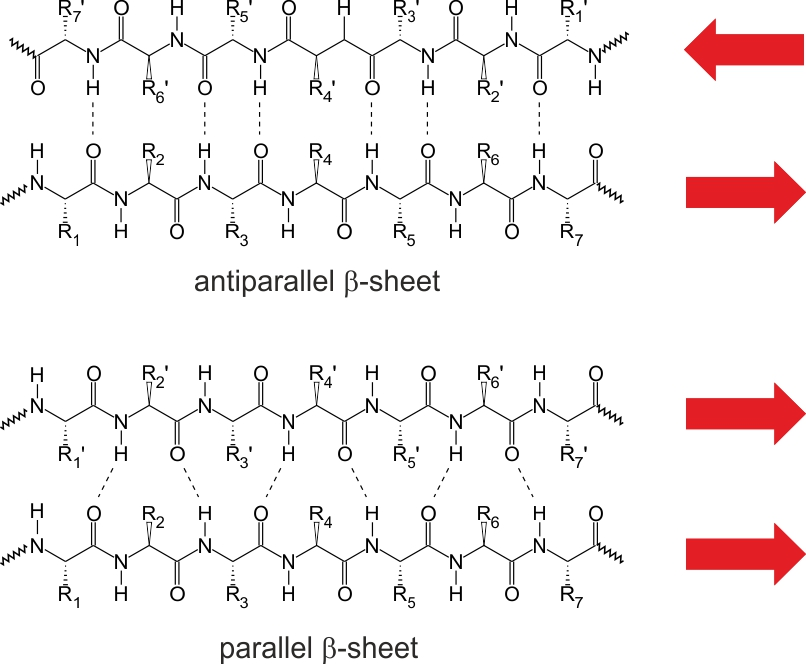
Q: What’s the difference between parallel and antiparallel β-sheets?
A: Antiparallel sheets are more stable due to optimal H-bond geometry. Parallel sheets require more strands for stability.
Q: What is a β-bulge?
A: A distortion in an antiparallel β-sheet where one strand has an extra residue, causing a bulge and gentle bend.
Q: What is a β-turn?
A: A 180° reversal in the peptide chain over 4 amino acids, stabilized by an H-bond between residues 1 and 4.
Q: What’s the difference between Type I and Type II β-turns?
A: Type I contains proline at position 2; Type II contains glycine at position 3 and no proline.
Q: What forces maintain secondary structure?
A: Hydrogen bonds between the carbonyl oxygen and amido hydrogen of the peptide backbone.
Q: Which groups stabilize secondary structure?
A: The main chain carbonyl and amido groups of the peptide bond (not side chains).
Q: What defines the tertiary (3°) structure of a protein?
A: Tertiary structure is the folding of a single polypeptide chain into a compact 3D shape, stabilized by long-range interactions among amino acid side chains.
Q: What intramolecular forces stabilize tertiary structure? (5)
Hydrophobic interactions
hydrogen bonds
salt bridges (electrostatic interactions)
hydration (structured water)
disulfide bonds.
Q: How do R group interactions maintain the tertiary structure? (4)
hydrophobic interactions (nonpolar side chains)
hydrogen bonds (polar or charged side chains)
salt bridges (acidic and basic side chains)
disulfide bonds (between cysteine residues).
Q: What are structural motifs in protein folding?
A: Common combinations of secondary structures that occur locally in proteins, such as helix-loop-helix, β-meander, and Greek key. They are not independently stable.
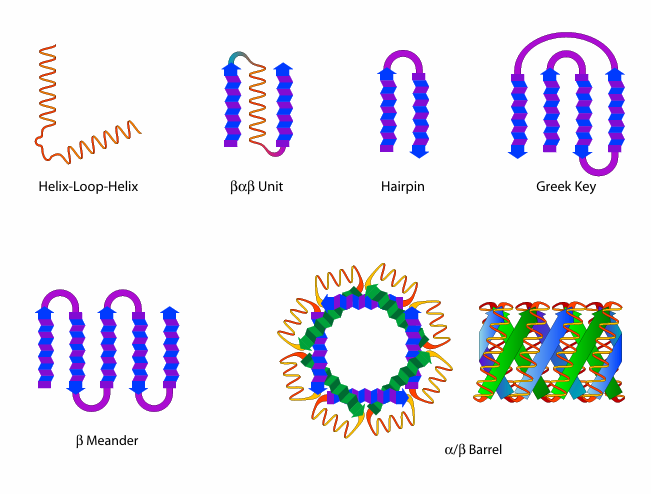
Q: What are domains in protein structure?
A: Independently folded, compact units within a protein’s tertiary structure that often have specific biochemical functions and can be stable on their own.
Q: What is the difference between motifs and domains?
A: Motifs are smaller, non-independent arrangements of secondary structures, while domains are larger, independently stable units that often have specific functions.
Q: What defines the quaternary (4°) structure of a protein?
A: The spatial arrangement and interactions between multiple polypeptide chains (subunits) to form a functional multimeric protein.
Q: What intramolecular forces stabilize quaternary structure? (5)
A: The same forces as tertiary structure
hydrophobic interactions
hydrogen bonds
salt bridges
hydration
sometimes disulfide bonds.
Q: How do R group interactions maintain quaternary structure?
A: By forming non-covalent interactions and sometimes disulfide bonds between side chains on different subunits, helping to maintain the overall protein complex.
Q: What are non-regular, non-repeating structures in proteins?
A: These are segments of polypeptide chains that lack regular α-helix or β-sheet secondary structure, often including loops or coils that connect structural elements.
Q: What are intrinsically unstructured or natively unfolded proteins?
A: Proteins or protein regions that do not adopt a defined 3D structure under physiological conditions but are still functional, often becoming structured upon binding to other molecules.
Q: What is the difference between non-regular non-repeating structures and intrinsically unstructured proteins?
A: Non-regular regions occur within otherwise folded proteins; intrinsically unstructured proteins remain disordered in isolation and often rely on partner interactions for structure.
Q: Why is entropy unfavorable during protein folding?
A: Folding reduces conformational freedom (entropy) of the polypeptide chain, favoring a random coil over a structured state.
Q: What enthalpic changes favor protein folding? (2)(3)
A: Intramolecular side chain interactions:
hydrogen bonds
ionic interactions
van der Waals forces
stabilize the folded state, lowering enthalpy.
Q: What is the hydrophobic effect in protein folding?
A: Hydrophobic side chains bury in the protein core, releasing structured water and increasing the entropy of the surrounding solvent, which favors folding.
Q: What is the postulated hierarchy of protein folding?
A: Protein folding is thought to follow a pathway: local secondary structures form first, followed by tertiary structure as these elements interact.
Q: What are chaperones?
A: Proteins that assist the folding of other proteins by preventing misfolding and aggregation.
Q: What are chaperonins?
A: A subclass of chaperones that provide an isolated environment for proteins to fold correctly, often barrel-shaped
Q: What is the difference between chaperones and chaperonins?
A: Chaperones generally prevent misfolding, while chaperonins actively assist folding in a confined space.
Q: What are fibrous proteins?
A: Long, insoluble proteins with structural roles (e.g., collagen, keratin), often composed of repeating sequences and dominated by secondary structures.
Q: What are globular proteins?
A: Compact, soluble proteins with diverse functions (e.g., enzymes, antibodies), containing mixed secondary and tertiary structures.
Q: What are membrane proteins?
A: Proteins associated with or embedded in cell membranes, often containing hydrophobic regions to interact with lipid bilayers.
Q: What is the difference between conjugated and unconjugated proteins?
A: Conjugated proteins have a prosthetic group, whereas unconjugated proteins do not.
Q: What are examples of prosthetic groups? (4)
Metals
heme
lipids
carbohydrates (O-linked and N-linked).
Q: What is the difference between an apoprotein and a holoprotein?
A: An apoprotein lacks its prosthetic group; a holoprotein includes it and is functionally complete.
Q: What is protein denaturation on a molecular level?
A: Denaturation is the disruption of secondary, tertiary, and quaternary structure without breaking peptide bonds, leading to loss of function.
Q: What is the difference between hydrolysis and denaturation?
A: Hydrolysis breaks peptide bonds (affecting primary structure); denaturation disrupts higher-order structures but leaves the primary sequence intact.
Q: What level(s) of structure does denaturation affect?
A: Secondary, tertiary, and quaternary structures.
Q: How does heat denature proteins?
A: Heat increases molecular motion, disrupting hydrogen bonds and hydrophobic interactions.
Q: How does physical agitation denature proteins?
A: Stirring or shaking introduces air-water interfaces that disrupt noncovalent interactions and protein structure.
Q: How do pH changes denature proteins?
A: Extreme pH alters charges on side chains, disrupting salt bridges and hydrogen bonds.
Q: How do detergents denature proteins?
A: Detergents insert into hydrophobic regions, disrupting the protein’s interior and surface interactions.
Q: How do organic solvents denature proteins?
A: Organic solvents alter the polarity of the environment, disrupting hydrophobic and hydrogen bonding.
Q: How do chaotropic agents like urea or guanidinium hydrochloride denature proteins?
A: They disrupt hydrogen bonding and solvate hydrophobic regions, destabilizing protein folding.
Q: How do heavy metals denature proteins?
A: Heavy metals bind to sulfhydryl groups and other side chains, disrupting tertiary and quaternary structure.
Q: What is the primary structure of collagen?
A: Repeating -Gly-Pro-X- or -Hyp-Gly-X- sequences
X is often lysine.
Hyp is 4-Hydroxyproline
Q: What is the name of the secondary structure of collagen? What does it look like?
Protropocollagen polypeptide. It contains an amino terminal globular domain, a left handed helix, and a carboxyl terminal globular domain.
Where is Protropocollagen transported? What happens next?
transported into the smooth endoplasmic reticulum where the addition of the hydroxyl group to proline and lysine occurs. The hydroxylation reactions requires ASCORBIC ACID (Vitamin C).
Q: What is the tertiary structure of collagen?
After enough proline residues have been hydroxylated, the triple helical structure will form starting at the associated carboxyl termini to the amino terminal globular domain. Heat Shock Protein is needed.
What is the name of the tertiary structure of collagen?
Tropocollagen
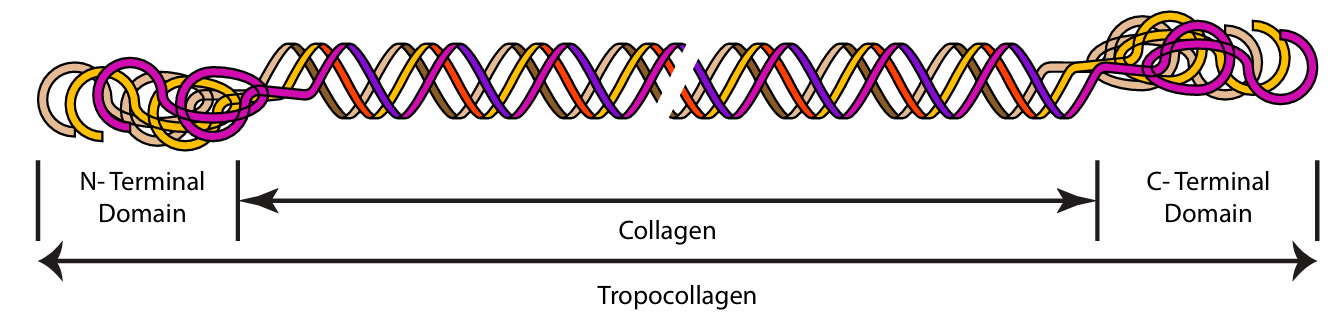
What happens to Tropocollagen in the extracellular space?
Enzymes cleave off domain to make collagen. The collagen Self-associate into staggered overlapping arrangement. Hydrophobic interactions hold helix together.
Q: What role does vitamin C play in collagen metabolism?
A: It’s a cofactor for hydroxylation of proline and lysine; deficiency leads to scurvy due to impaired collagen stability.
Q: What is the function of myoglobin?
A: Myoglobin stores O₂ in muscle tissue by reversibly binding oxygen.
Q: What is the function of hemoglobin?
A: Hemoglobin transports O₂ from the lungs to tissues, reversibly binding four oxygen molecules.
Q: What is the quaternary structure of hemoglobin?
A: Hemoglobin is a heterotetramer with two α and two β subunits, each containing a heme group.
Q: How many heme groups are present in hemoglobin and myoglobin?
A: Hemoglobin has four heme groups (one per subunit); myoglobin has one heme group.
Q: What structural change occurs in prion diseases?
A: The prion protein transitions from having very little beta-sheet structure to a high beta-sheet content.
Q: Why are prion proteins resistant to denaturation and proteolysis?
A: Their high beta-sheet structure makes them highly stable and resistant to degradation.
Q: How do abnormal prion proteins affect normal proteins?
A: Abnormal prions bind to normal proteins and induce them to refold into the abnormal, beta-rich form.
Q: What is the consequence of prion protein accumulation in the brain?
A: It leads to neurodegenerative disorders due to protein aggregation and cell death.