Predictions from electrode potentials
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
If a redox system had a more positive Eo value what does this mean?
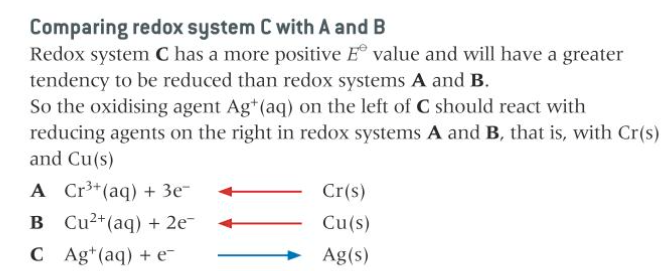
the oxidising agent on the left will react with the reducing agents on the right of the other redox systems
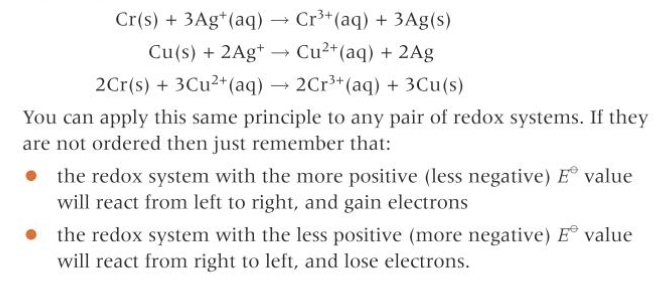
How do you write overall equations?
combing the half equations:
redox system with more positive Eo value will react from left to right
redox system with less positive Eo value will react from right to let
What are the limitations of predicting using Eo values?
give no indication of the rate of the reaction - may have large activation energy
actual conditions may be different from the standard conditions
standard elctrode potentials apply to aqueous equilbiria
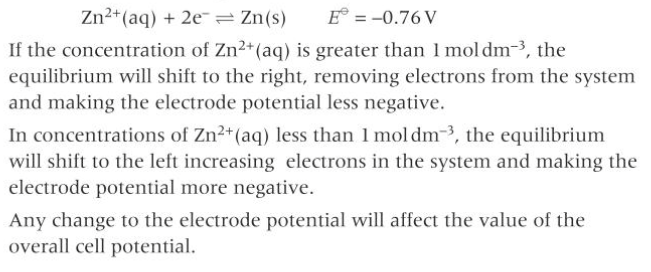
Why is concentration a limitation of predicting using Eo values?
standard electrode potentials are measured using 1mol dm-3
changing concentrations will cause equilibrium to shift and make electrode potentials more or less negative
When is a reaction feasible?
the standard cell potential, Eocell, is positive