Structure 3.2: Functional groups: Classification of organic compounds
1/114
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
115 Terms
Structural formula
A representation of the molecule showing how the atoms are bonded to each other.
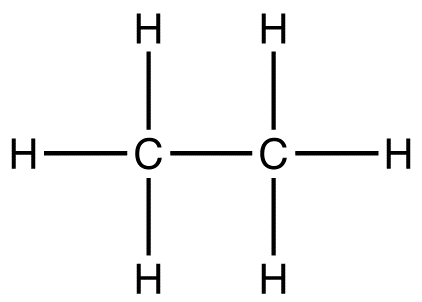
Full structural formula
Shows every bond and atom.
Condensed structural formula
Often omits bonds where they can be assumed, and groups atoms together.
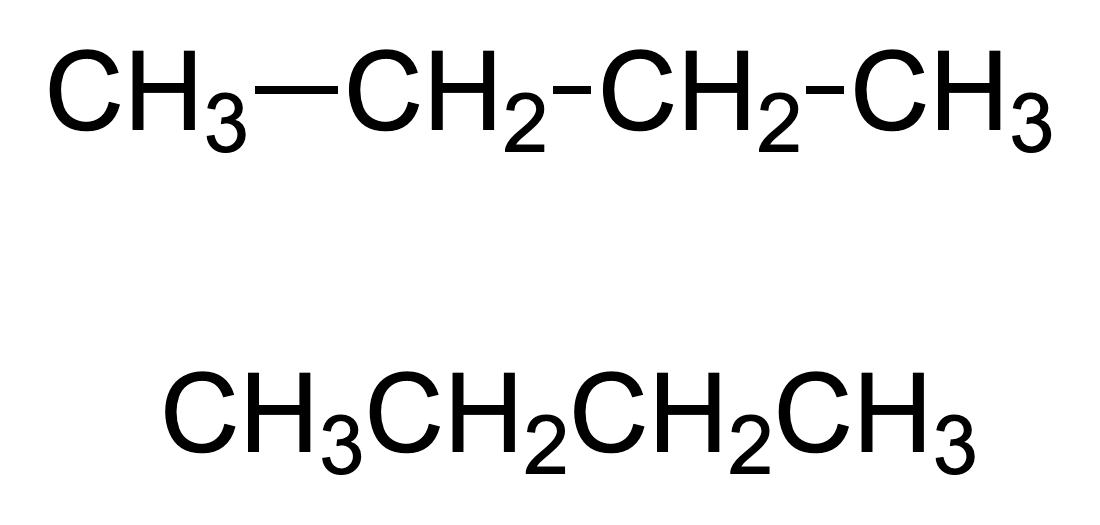
Condensed structural formula of ethane
CH3CH3
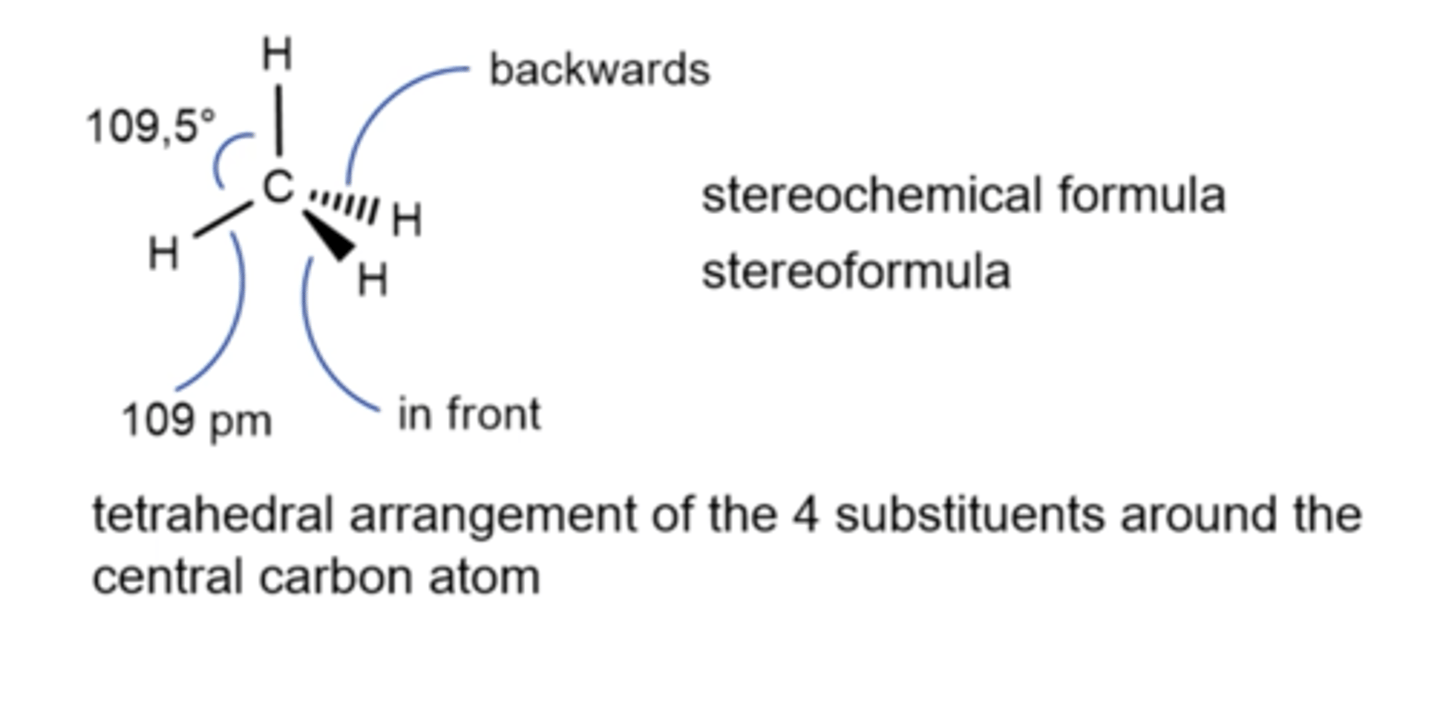
A stereochemical formula
Attemps to show the relative positions of atoms and groups around a central carbon in 3 dimensions
Functional groups
Atoms, or groups of atoms, that are present in organic compounds and are responsible for a compound's physical properties and chemical reactivity.
What functional group do carboxylic acids contain
-COOH
Alkyne general formula
CnH2n-2
Alkyne functional group
Alkynyl
Alkene functional group
Alkenyl
Alcohol general formula
CnH2n+1OH
P.g.348-349
Reaction pathway
Each step involved a functional group interconversation
Condensation reaction
A molecule of water is eliminated and a new bond is formed between the acid group of one amino acid and the amoino group of the other.
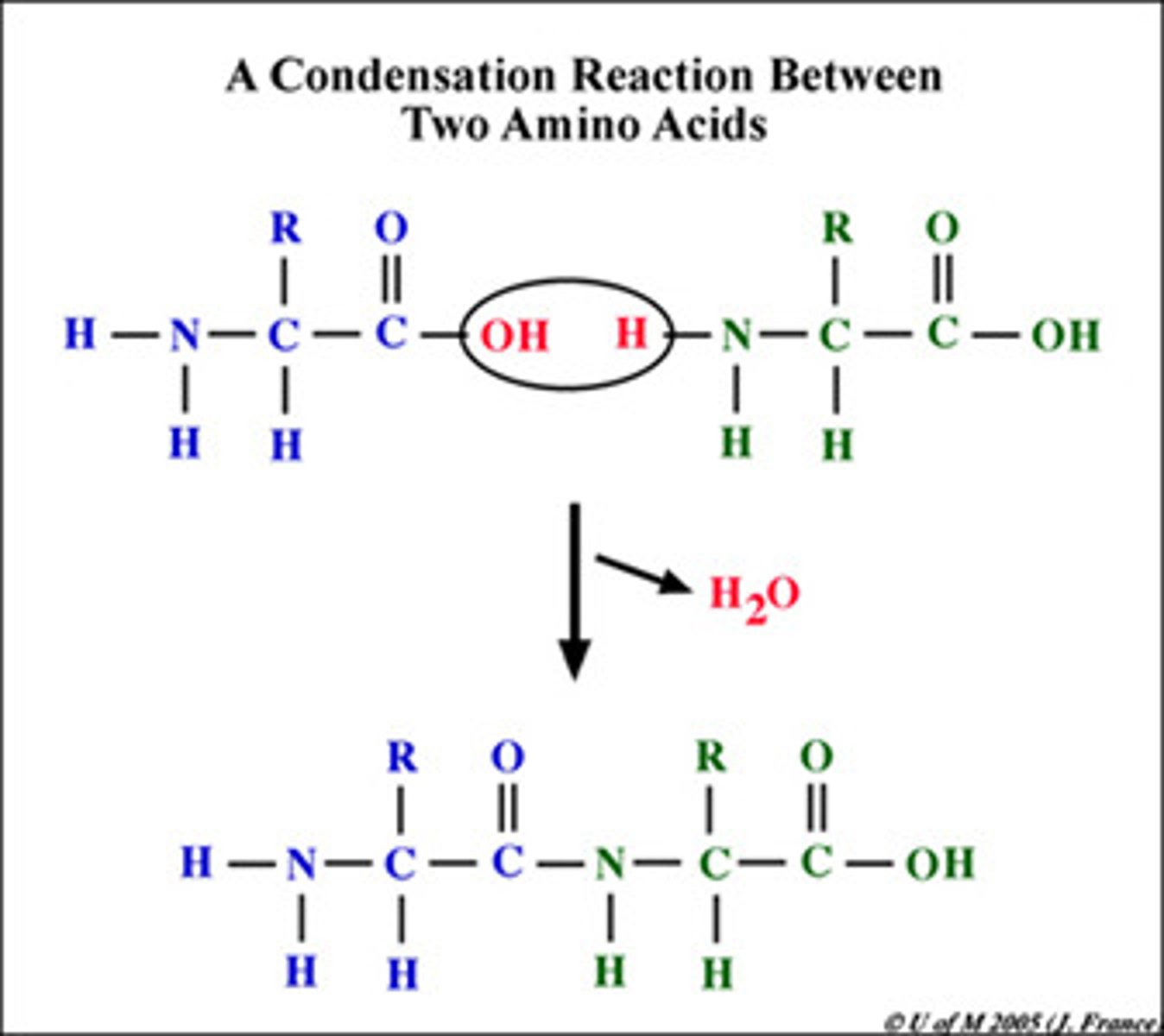
Amide link
The bond substituded in a condensation reaction (N-H)
Peptide bond
The bond that is a substituted amide link
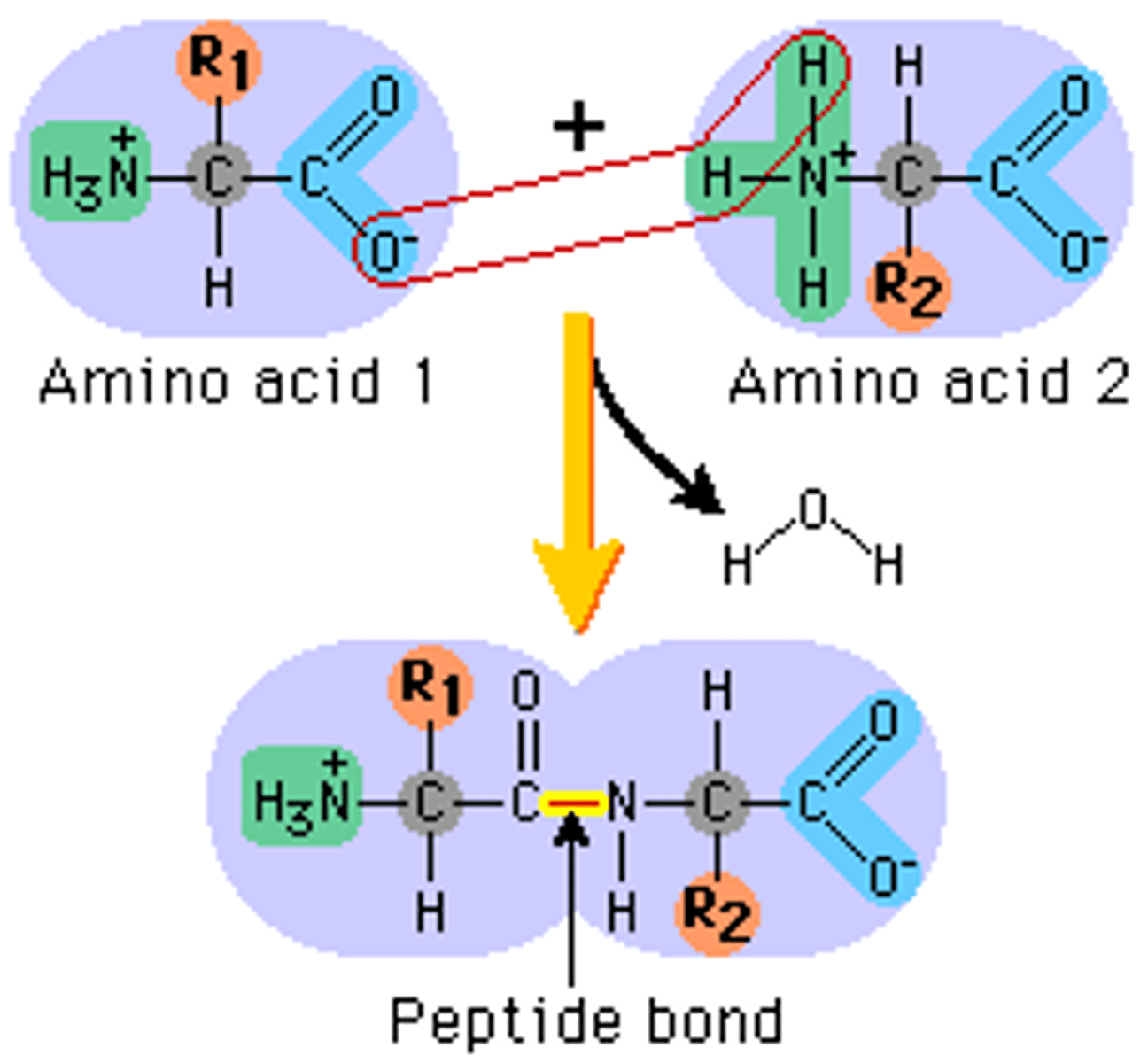
Dipeptide
Two amino acids bonded together
Tripeptide
Three amino acids bonded together
Homologous series
A series of organic compounds with the same functional group but with each successive member differing by CH2
Most volatile to least volatile
Alkane>halogenoalkane>aldehyde>ketone>alcohol>carboxylic acid
Increasing strength of intermolecular attraction
London (dispersion) force → dipole-dipole interaction → hydrogen bonding
Increasing boiling point
London (dispersion) force → dipole-dipole interaction → hydrogen bonding
Main intermolecular attraction in alkanes
London (dispersion) force
Main intermolecular attraction in halogenoalkanes, aldehydes and ketones
Dipole-dipole interaction
Main intermolecular attraction in alcohols and carboxylic acids
Hydrogen bonding
Stem
Name for the longest chain of carbon atoms (e.g. meth-, eth-, prop-, etc.)
Rules for IUPAC nomenclature
1. Identify the longest straight chain of carbon atoms
2. Identify the functional group
3. Identidy the side chains or substituent groups
Distinction between class and functional group
Class refers to the type of compound and functional group which refers to the site of reactivity in the molecule.
Functional group of amine
-NH2
Functional group of halogenoalkane
-F, -Cl, -Br, -I
When do esters form?
When the alkyl group of an alcohol replaces the hydrogen of a carboxylic acid in a condensation reaction.
Example of ester reaction
R-COOH + R'OH → R-COO-R' + H2O
Where does the stem comes from in esters?
The parent acid
What is the prefix for esters?
The alkyl group
The ester made from the reaction of ethanol with ethanoic acid
Ethyl ethanoate
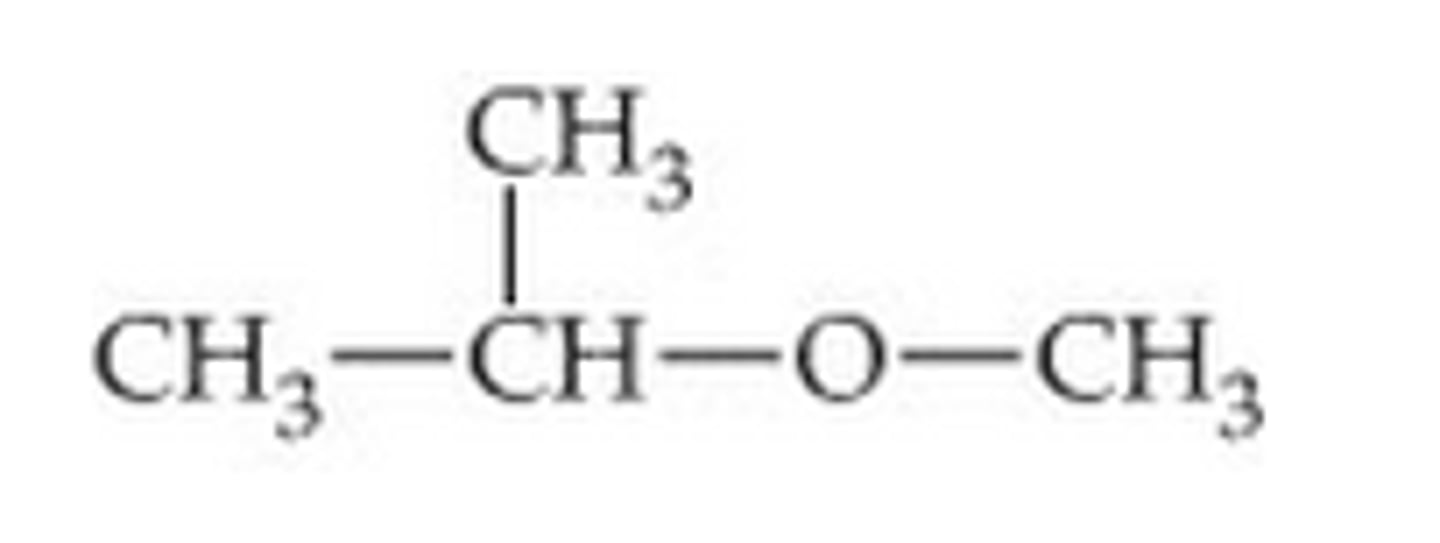
What do ethers consist of
2 alkyl chains linked by an oxygen atom
When naming ethers
-The longer chain will be the stem and retains its alkane name
-The shorter chain is regarded as a substituenet and is given the prefix alkoxy
Methoxypropane
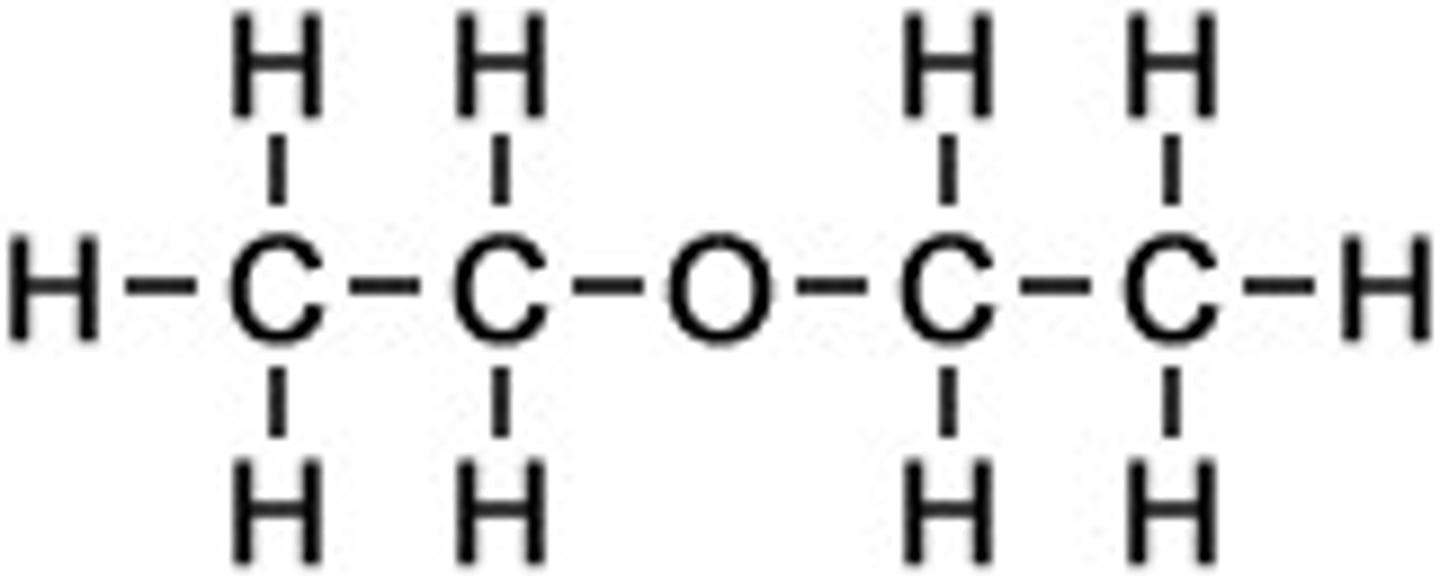
When two chains are the same length for ethers
One is assigned as the alkane stem and the other becomes the substituent
Ethoxyethane
Prefix
Position, number and name of substituents
Suffix
Class of compound determined by functional group
Structural isomers
Molecules that have the same molecular formula but different arrangements of the atoms. Because of their different structures, they also have different physical and chemical properties.
How does more branching affect the boiling point in an isomer?
Lowers the boiling point
Why does more branching affect the boiling point in an isomer?
It influences the strength of the London dispersion forces occurring between neighbouring molecules because it reduces the amount of surface contact. Branched-chain isomers have less contact with each other than their0single chain isomers resulting in weaker attractions between instantaneous and indeuced dipoles on neighbouring molecules with weaker overall London dispersion forces and lower boiling points.
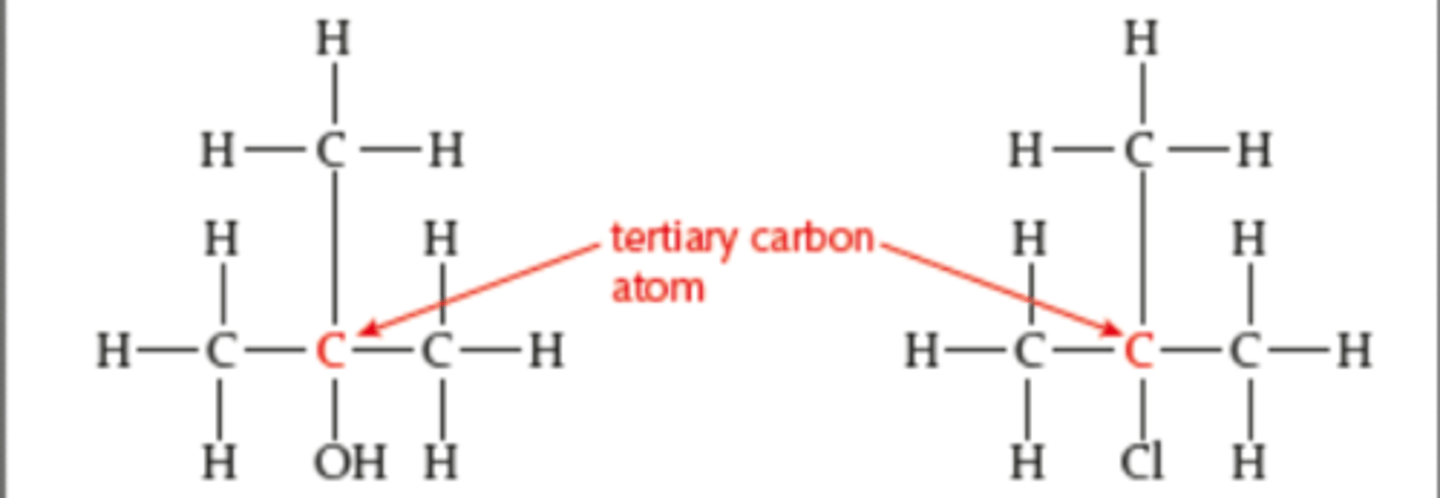
Primary molecules
A primary carbon atom is attached to the functional group and also to at least two hydrogen atoms.
Secondary molecules
A secondary carbon atom is attached to the functional group and also to one hydrogen atom and two alkyl groups.

Tertiary molecules
A tertiary carbon atom is attached to the functional group and is also bonded to three alkyl groups and so has no hydrogen atoms.
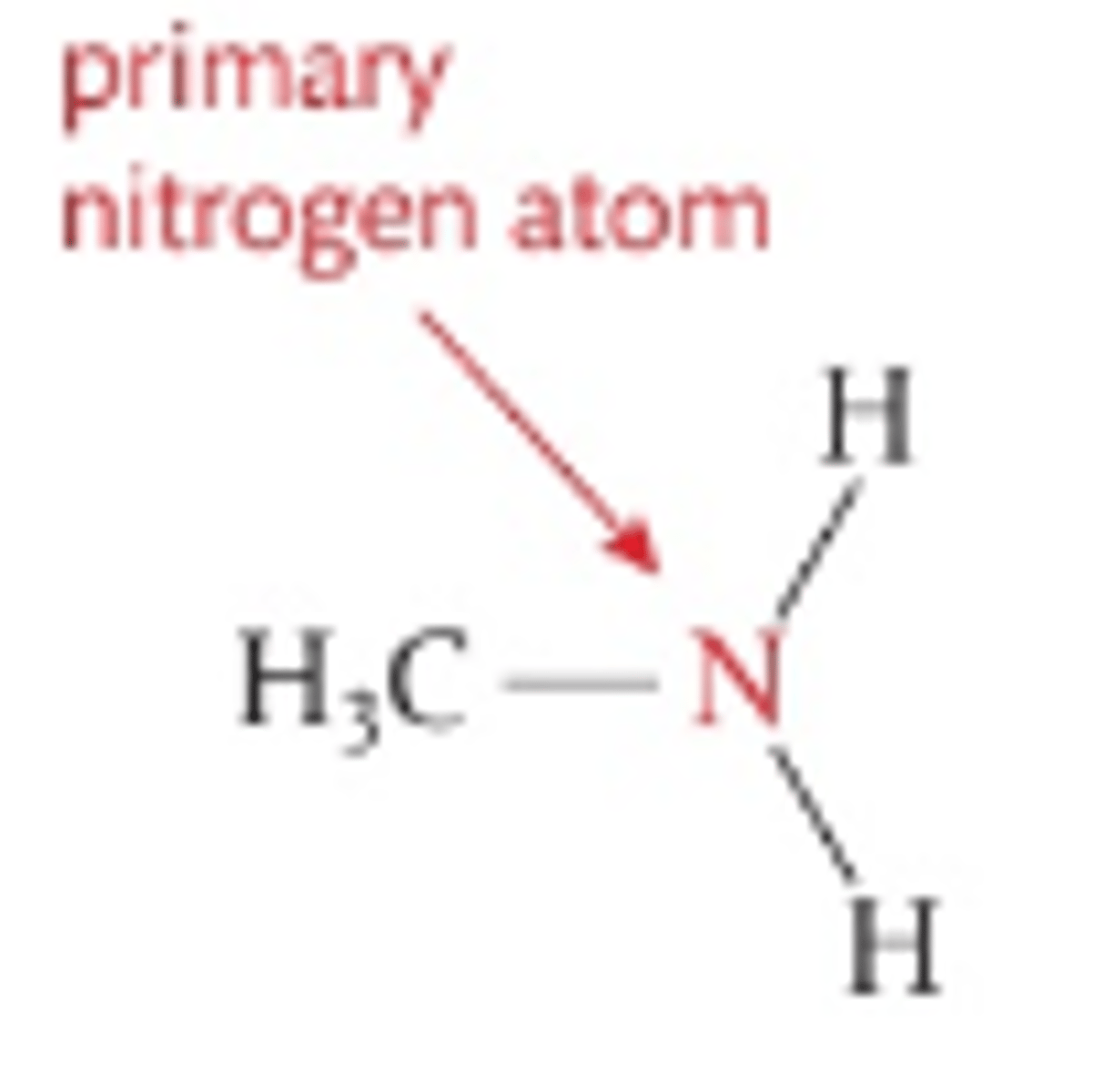
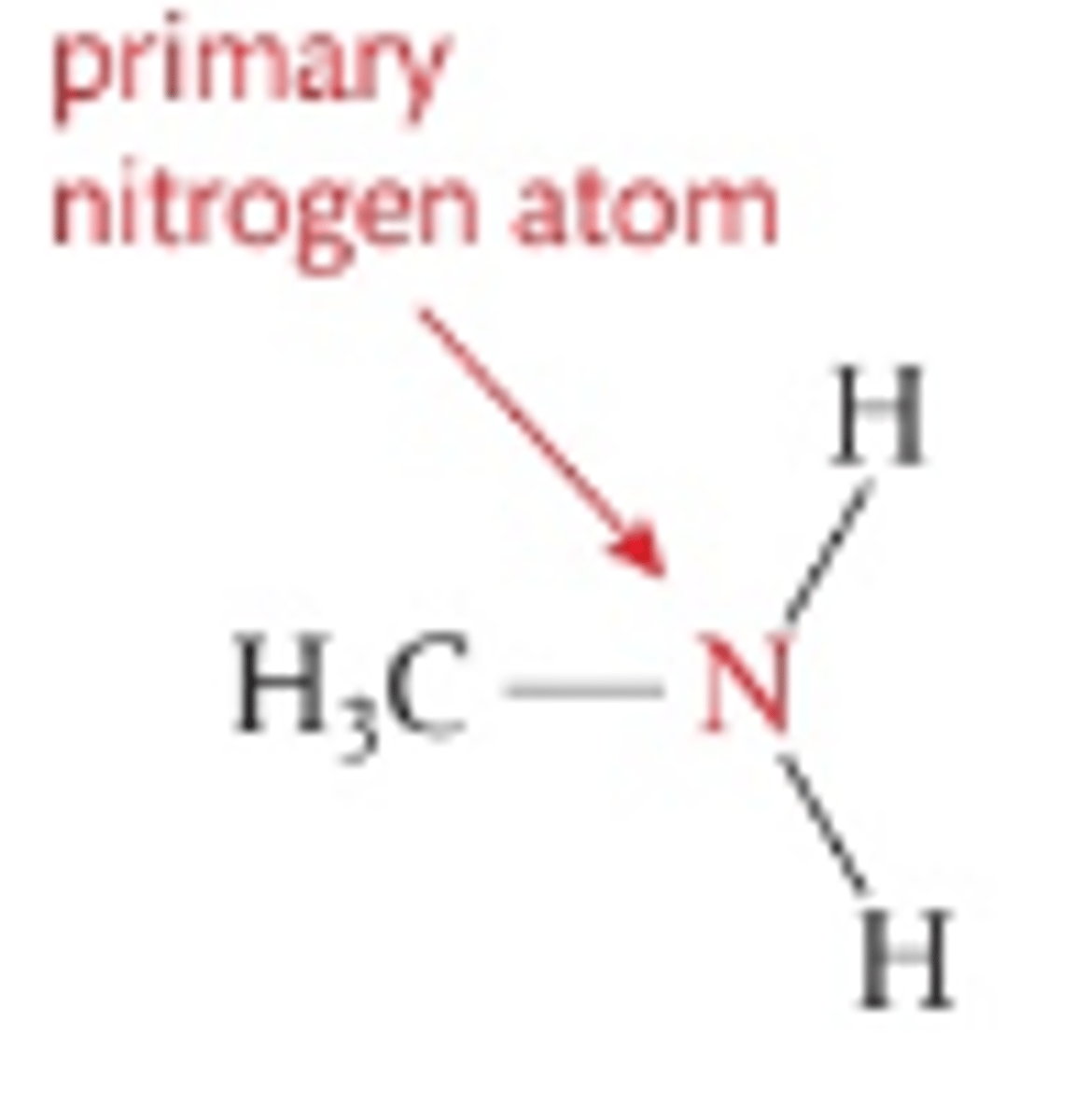
Number of H atoms attacthed to central N in primary amines
2
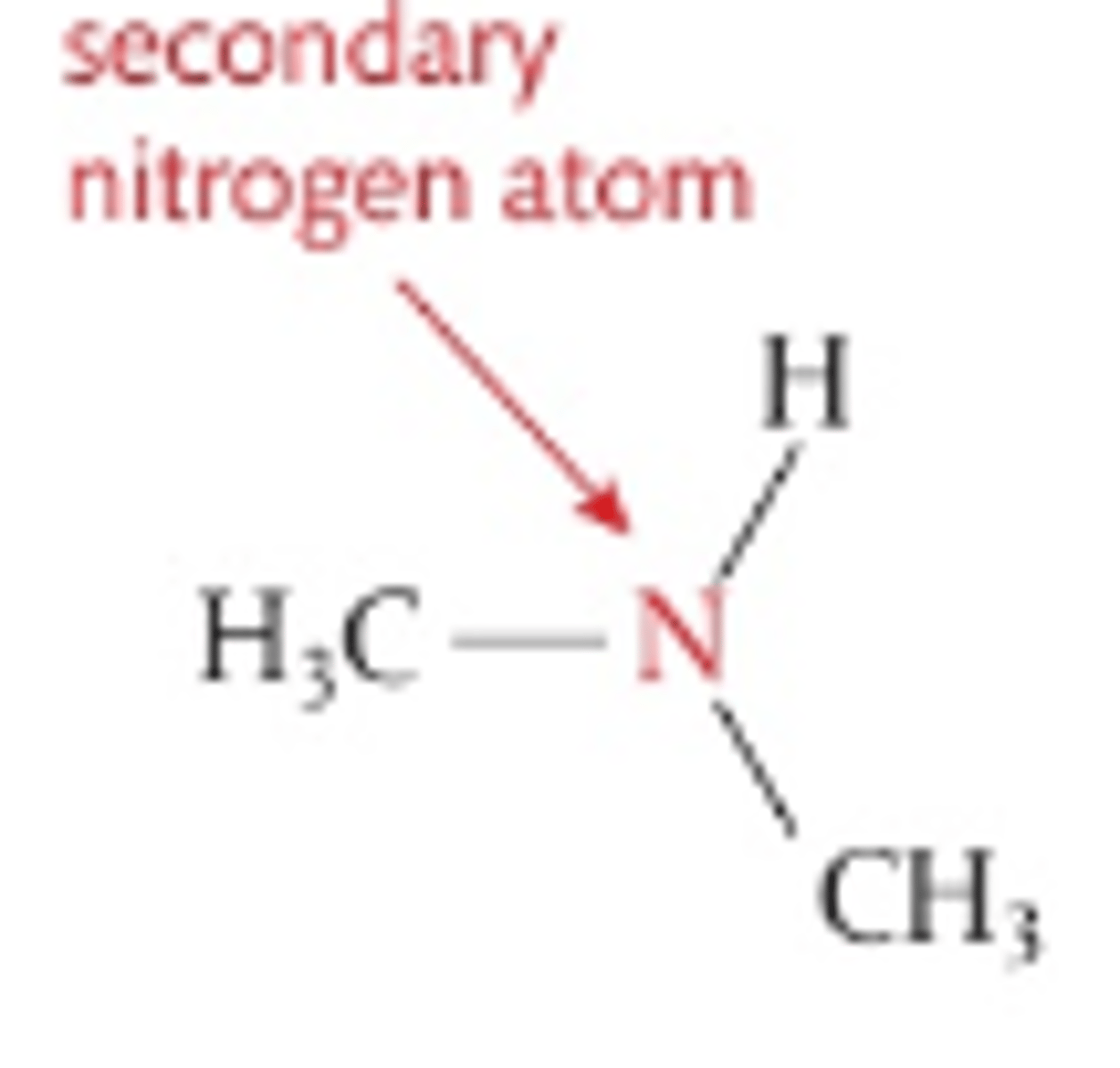
Number of H atoms attacthed to central N in secondary amines
1
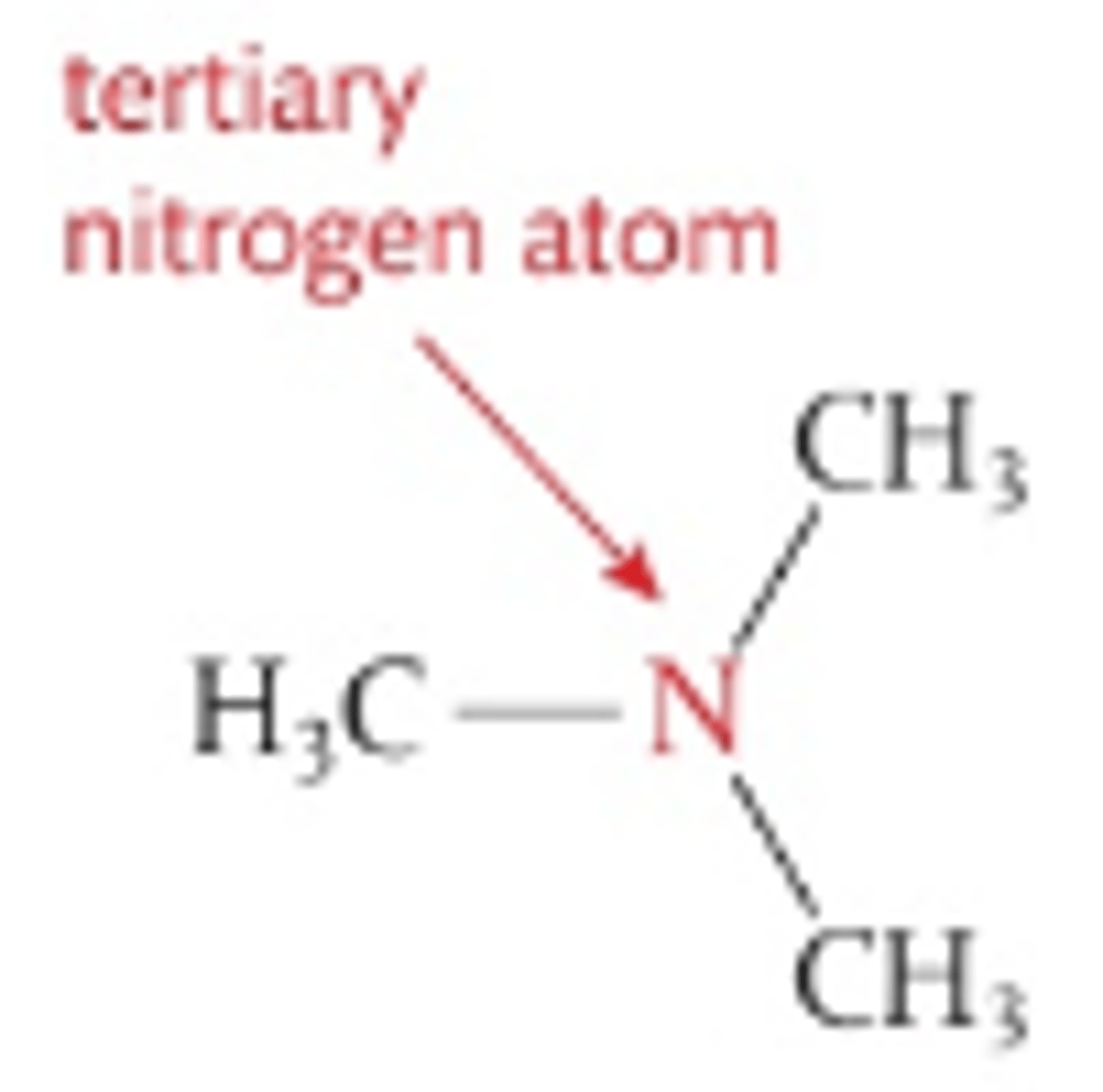
Number of H atoms attacthed to central N in tertiary amines
0
Number of C atoms attached to central N in primary amines
1
Number of C atoms attached to central N in secondary amines
2
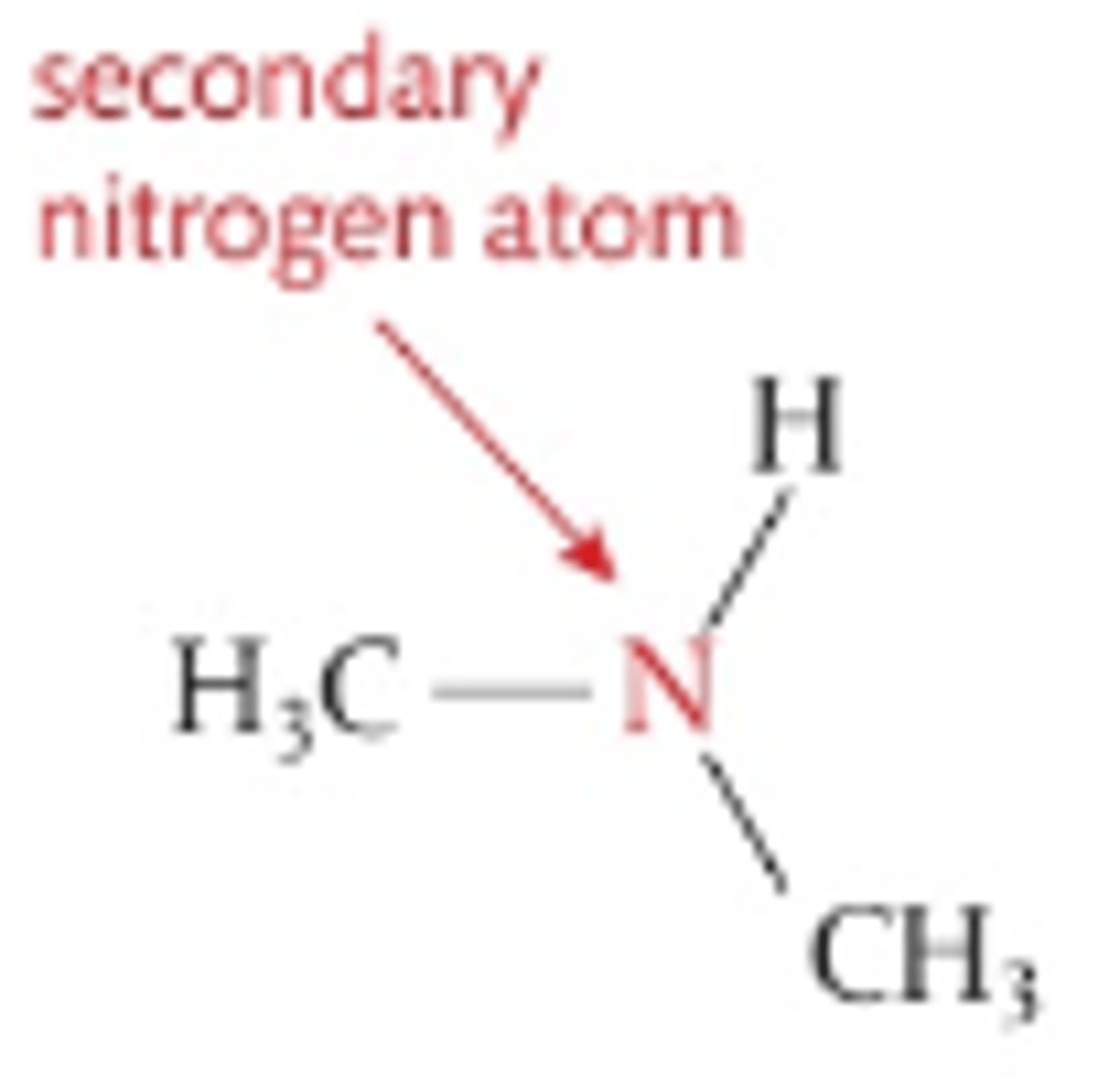
Number of C atoms attached to central N in tertiary amines
3

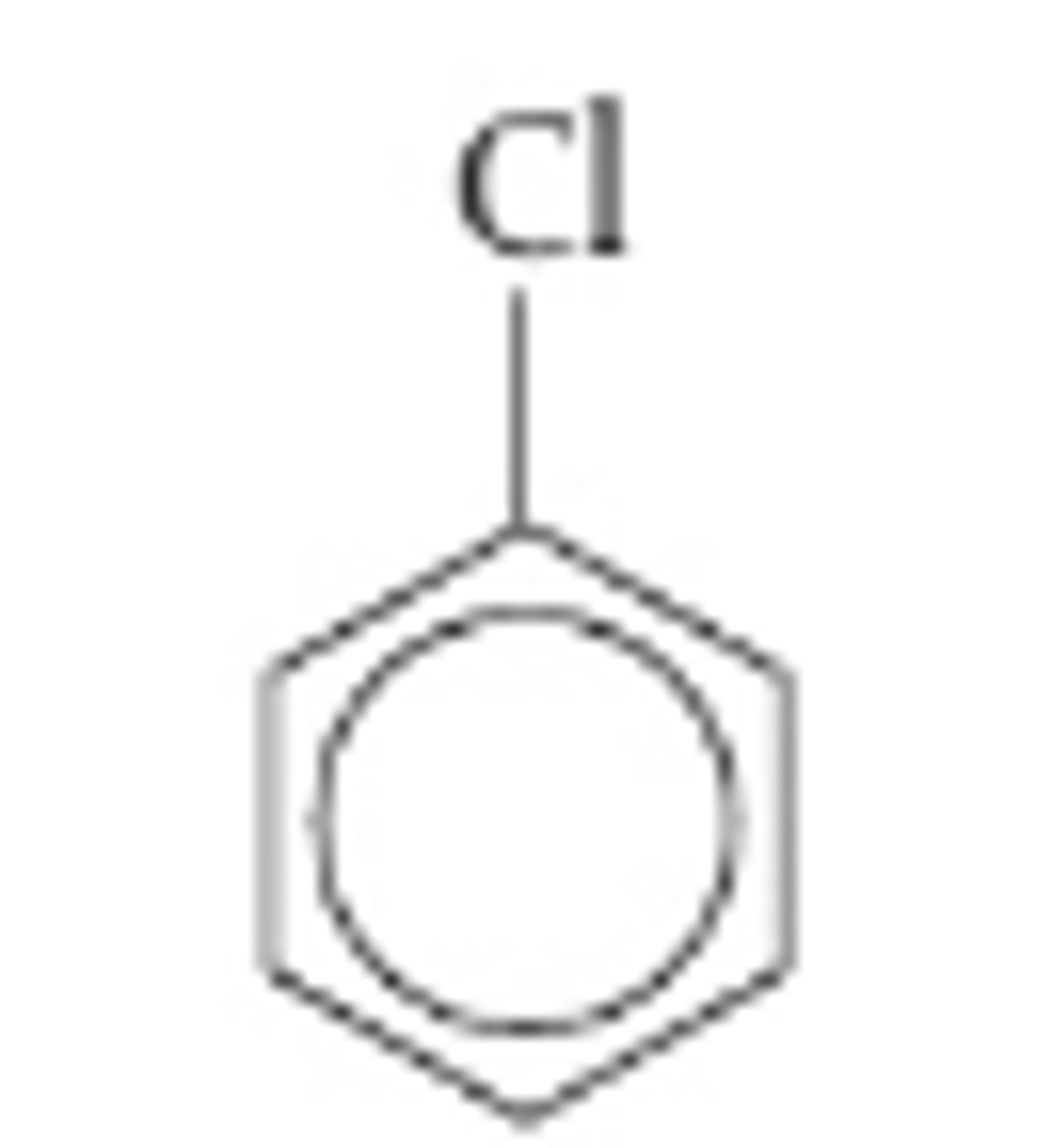
Chlorobenzene
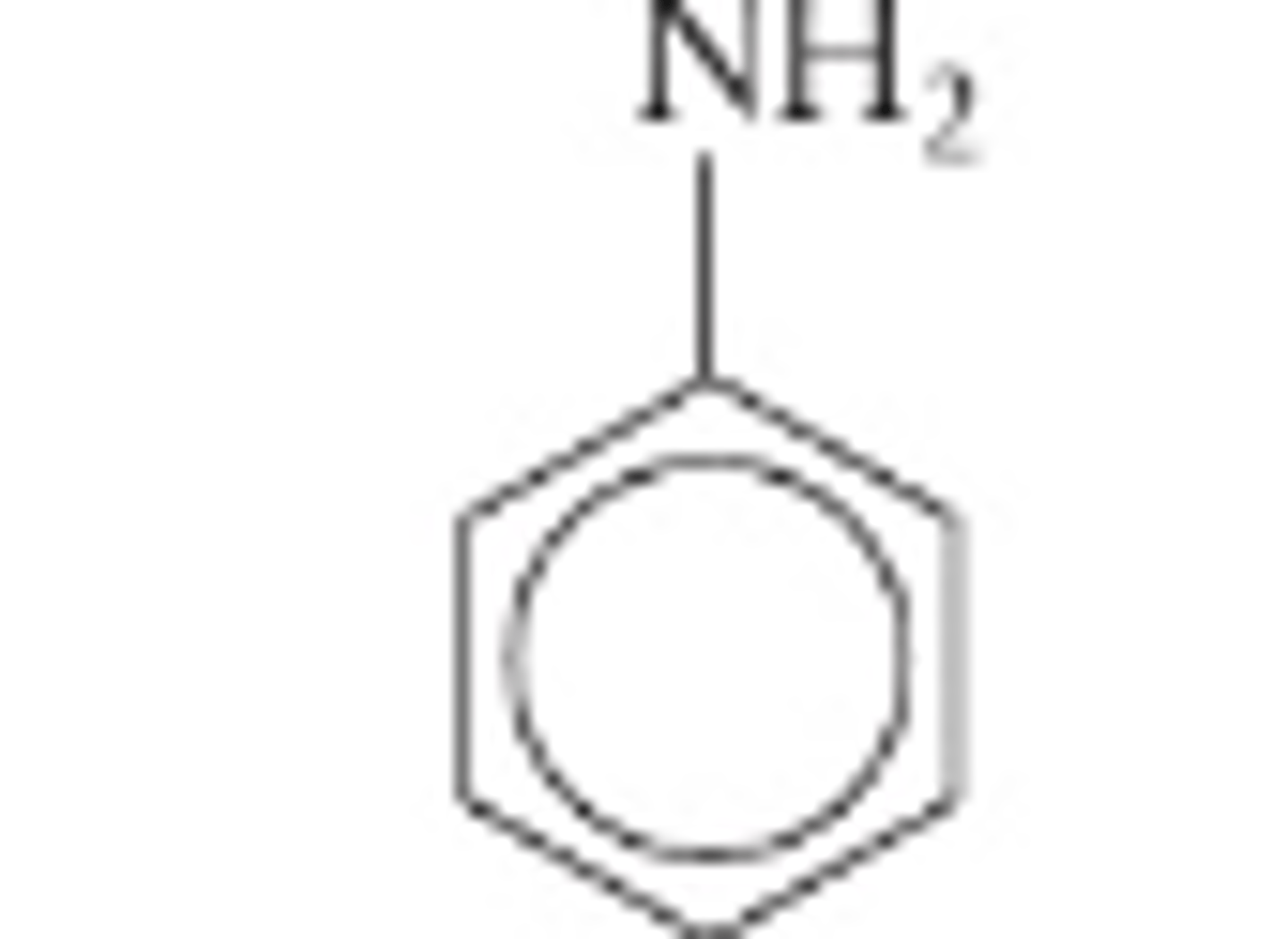
Phenylamine
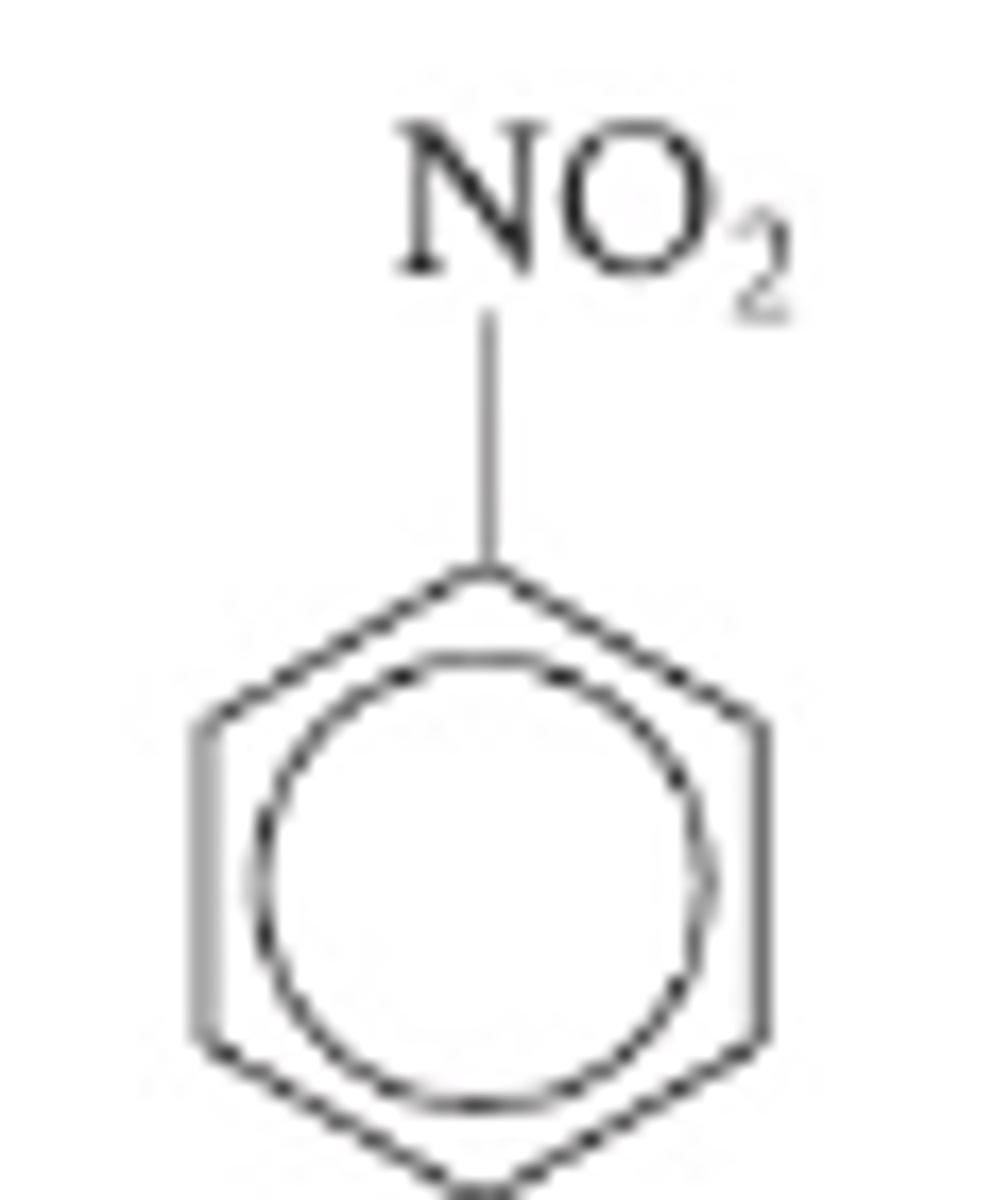
Nitrobenzene
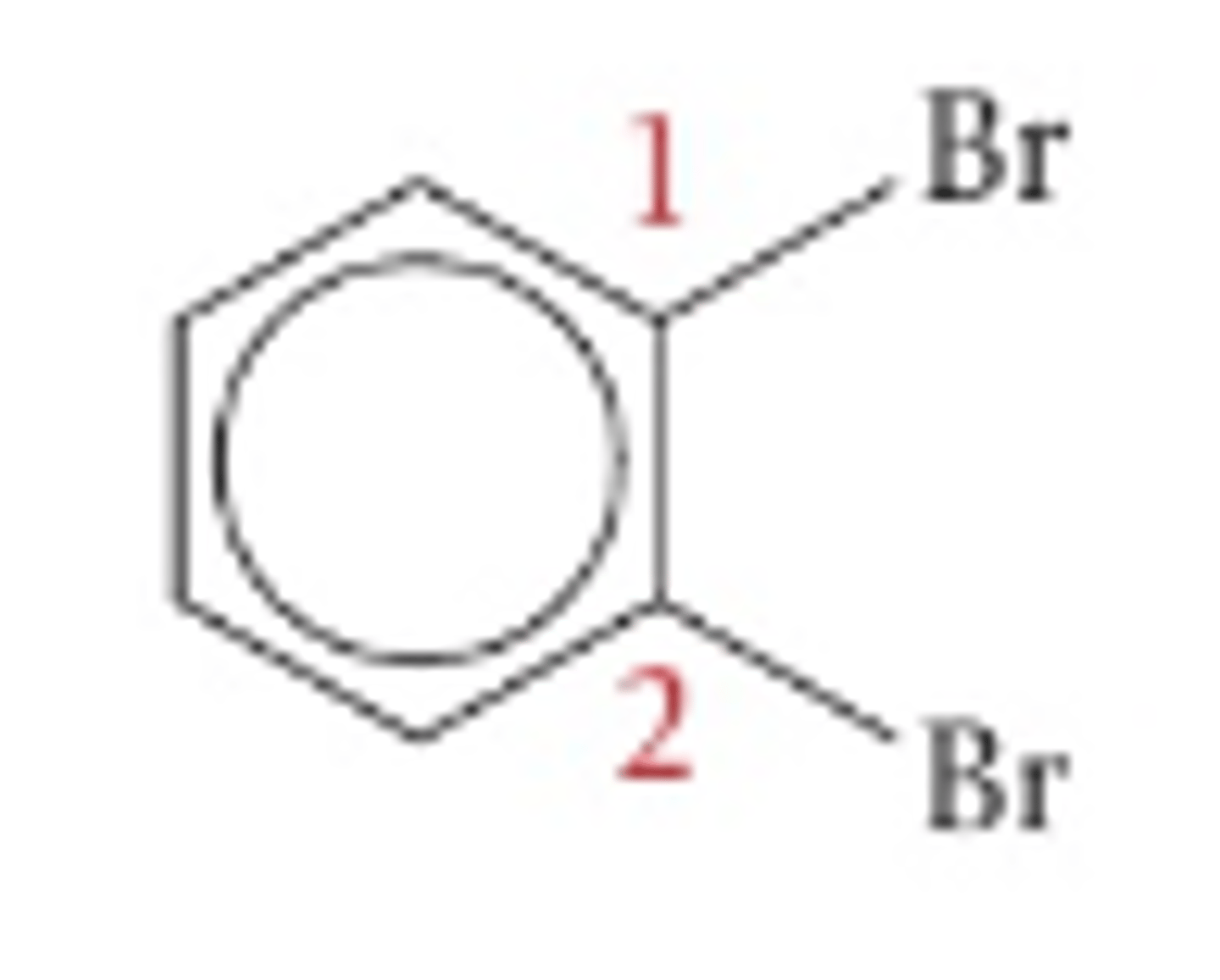
1,2-dibromobenzene
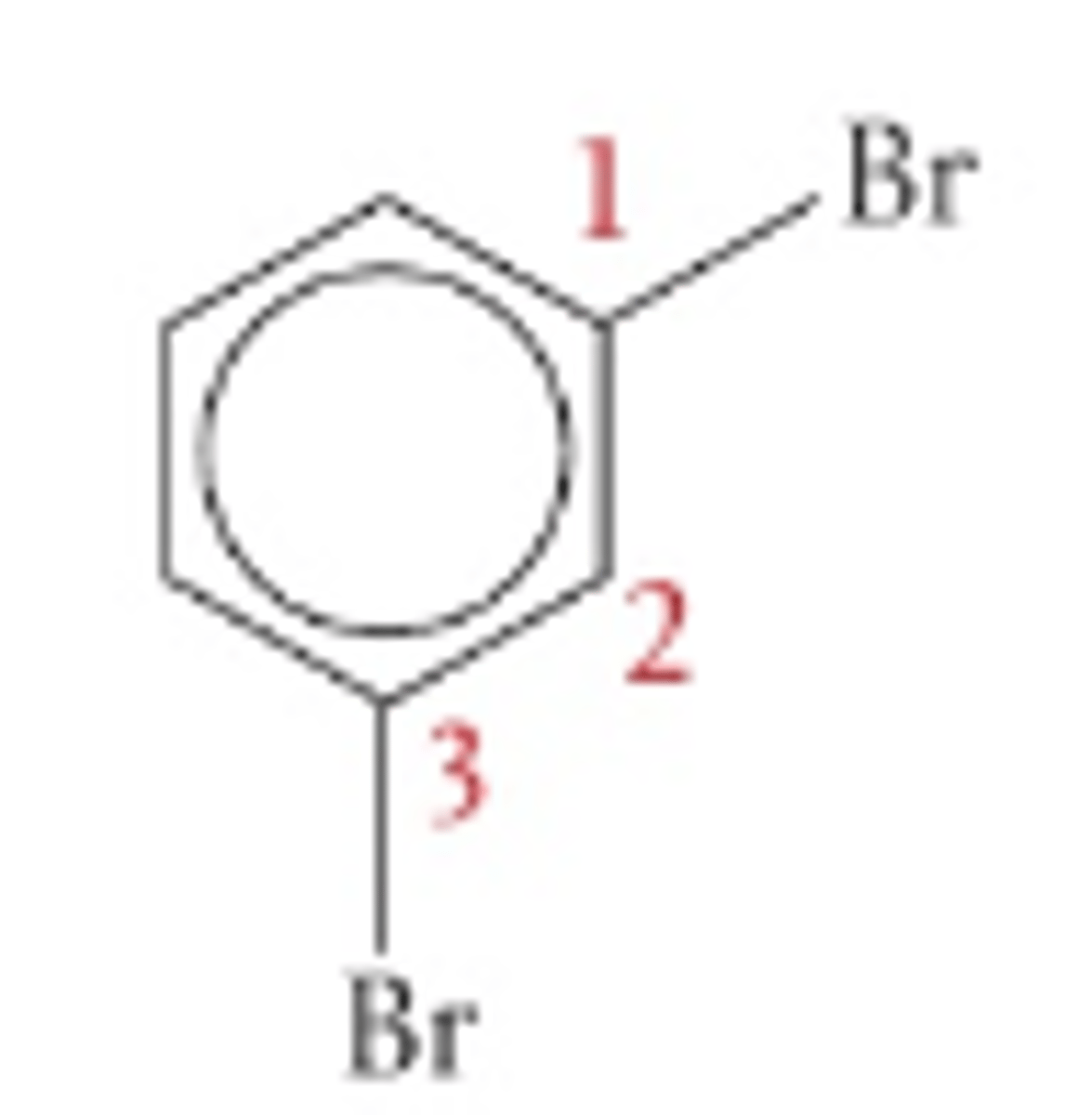
1,3-dibromobenzene
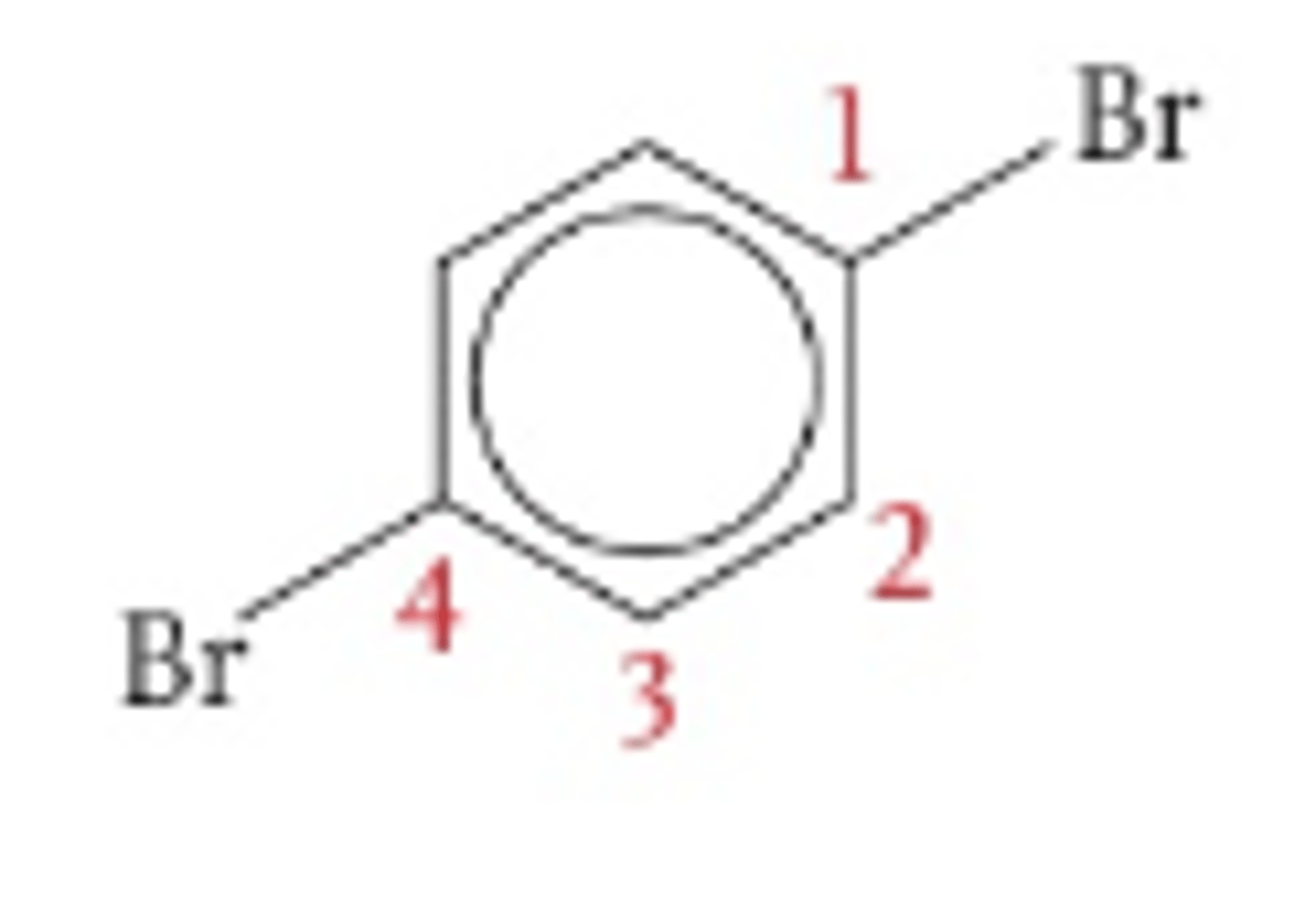
1,4-dibromobenzene
How are the numbers assigned to the ring positions of subsituted benzene
They are done so that the lowest possible are obtained
Isomerism
Compounds with same molecular formula but different arrangemetns of the atoms
Structural isomerism
Atoms and functional groups attached in different ways
Stereoisomerism
Different spatial arrangements of atoms in molecules
Configurational isomerism
Can be interconverted only by breaking covalent bonds
Conformational isomerism
Can be interconverted by free rotation of sigma bonds
Cis-trans isomerism
Exists where there is restricted rotation around atoms
Optical isomerism
Chirality exists where there is an asymmetric carbon atom
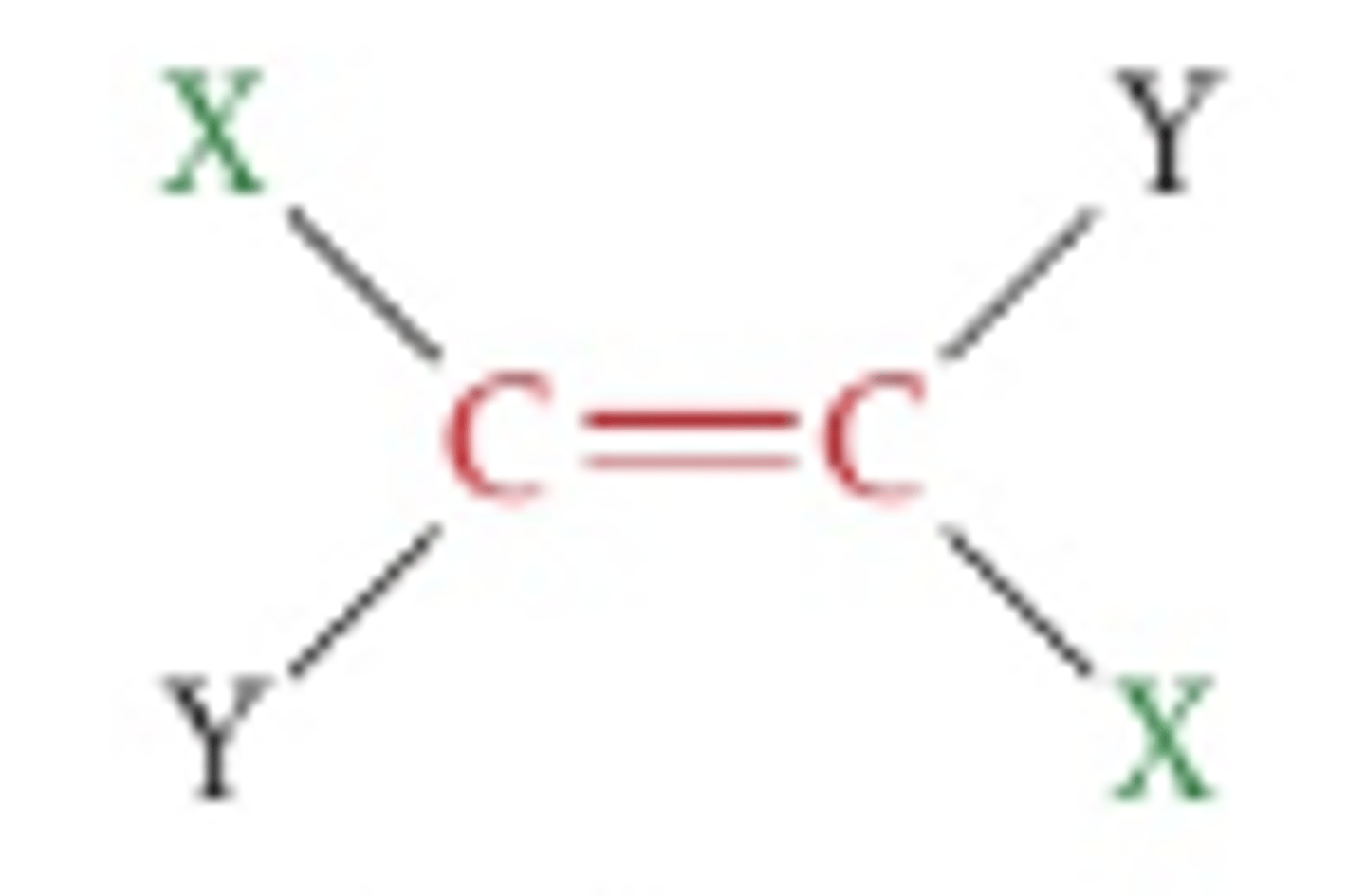
What do double bonds consist of?
One sigma bond and one pi bond, withthe pi bond forming by sideays overlap of two pi orbitals. The refernce plane is perpendicular to the sigma bonds and passes through the double bond.
Cis isomer
Refers to the isomer that has the same groups on the same side of the double bond/ring.
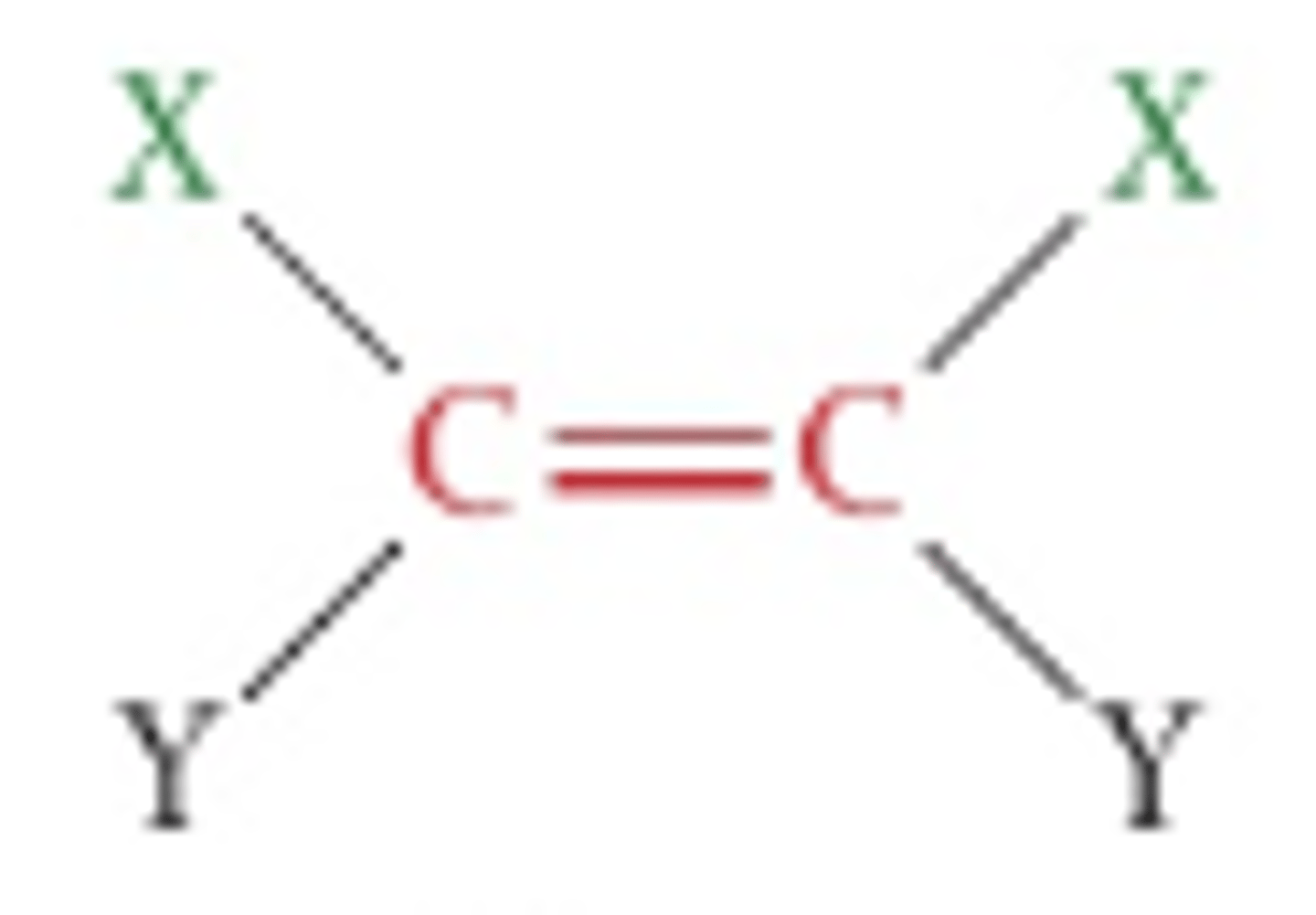
Trans isomer
Isomer that has the same groups on opposite sides, or across the reference plane.
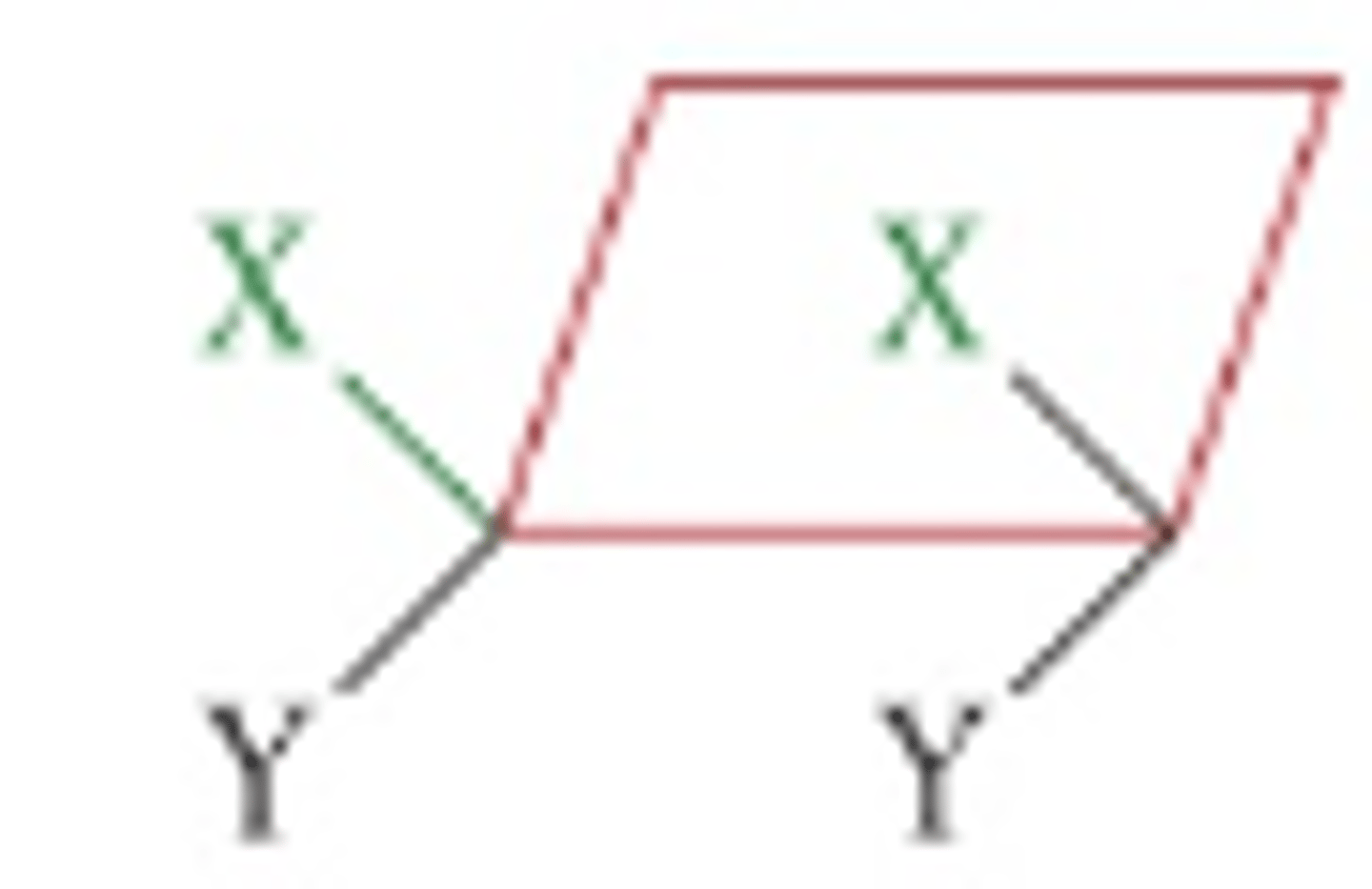
\Cycloalkanes
Contain a ring of carbon atoms that restricts rotation. The bond angles are stranied from the tetrahedral angles in the parent alkane.
Cis isomer for cyclic molecules
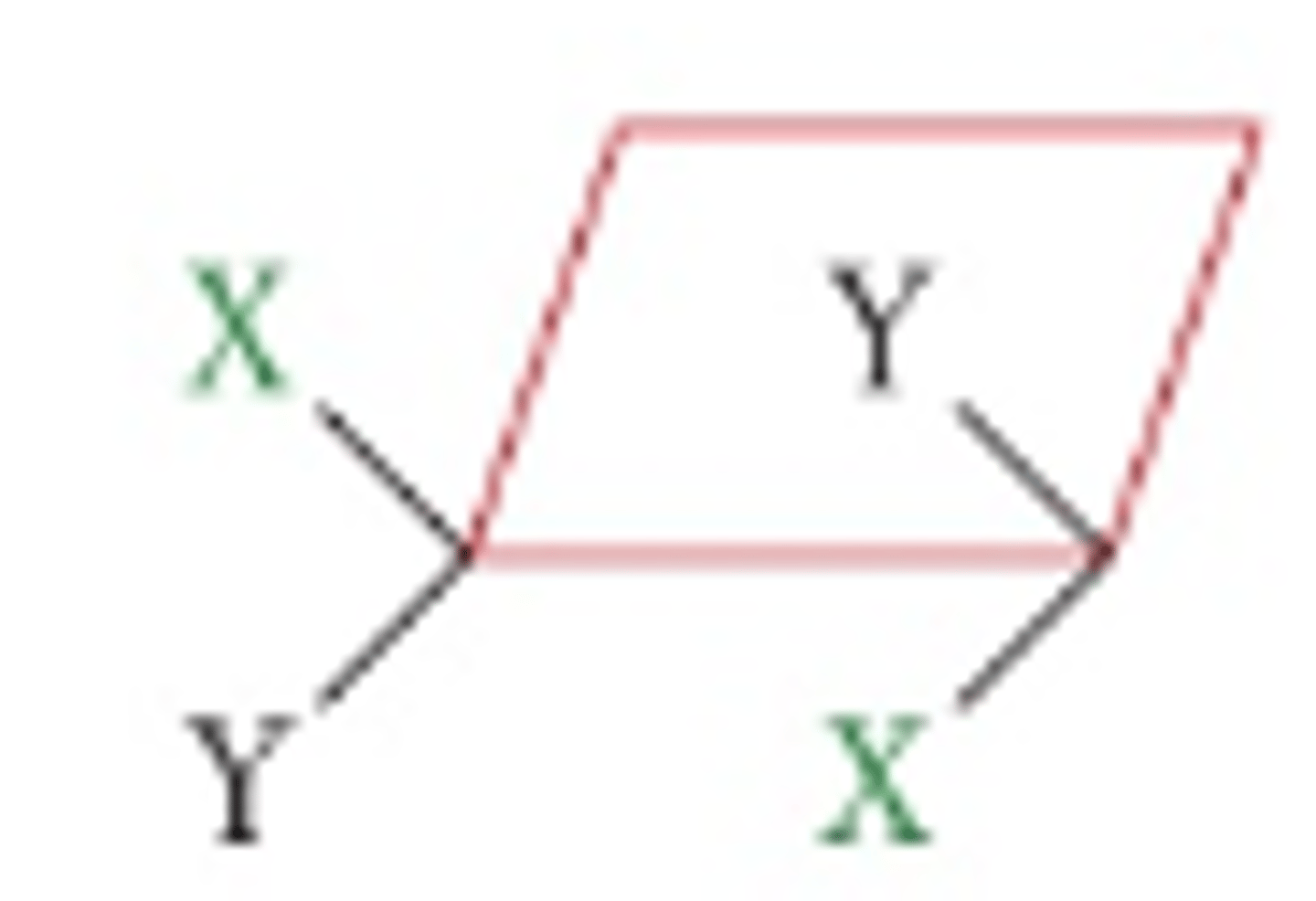
Trans isomer for cyclic molecules
Chiral
A carbon atom attached to 4 different atoms or groups.
Alternative names for chirals
Asymmetric or a sttereocentre
Optical isomerism
When the four groups, arranged tetrahedrally around the carbon atom with bond angles of 109;5 are arranged in two different three-dimensional configurations which are mirror images of each other.
Enantiomers:
identical physical properties except they rotate the plane of plane-polarised light in opposite directions
chemical properties are same except when they react with with other chiral molecules
mirror images
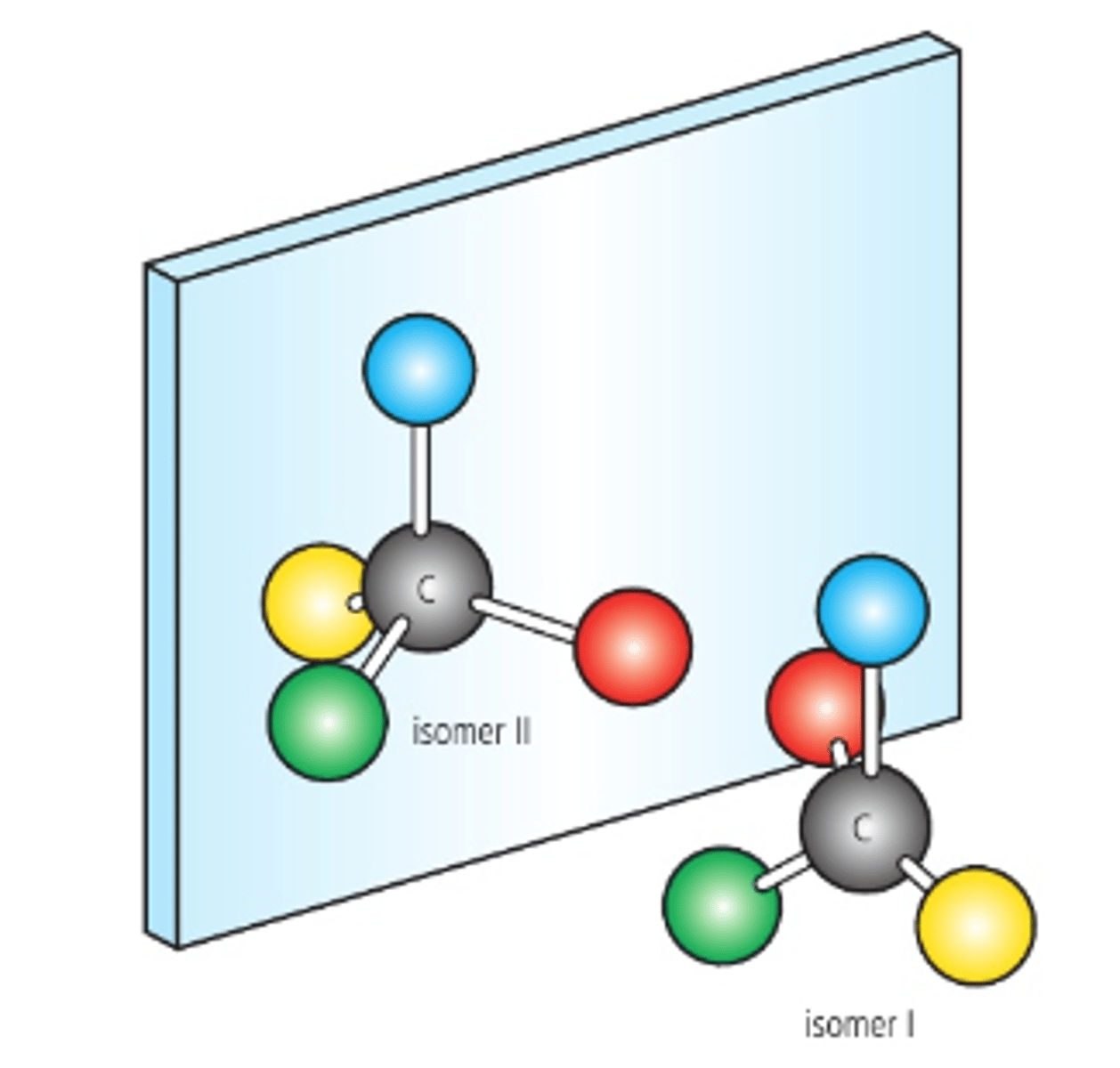
Non-superimposable
This means that the molecules cannot be placed on top of one another and give the same molecule.
Disastereomers
Molecuels that have different configurations at some, but not all, chiral centres. These molecules are not mirror images of each other.
Polarimeter
Measures the direction of rotation when a beam of plane-polarized light passes through a solution of optical isomers.
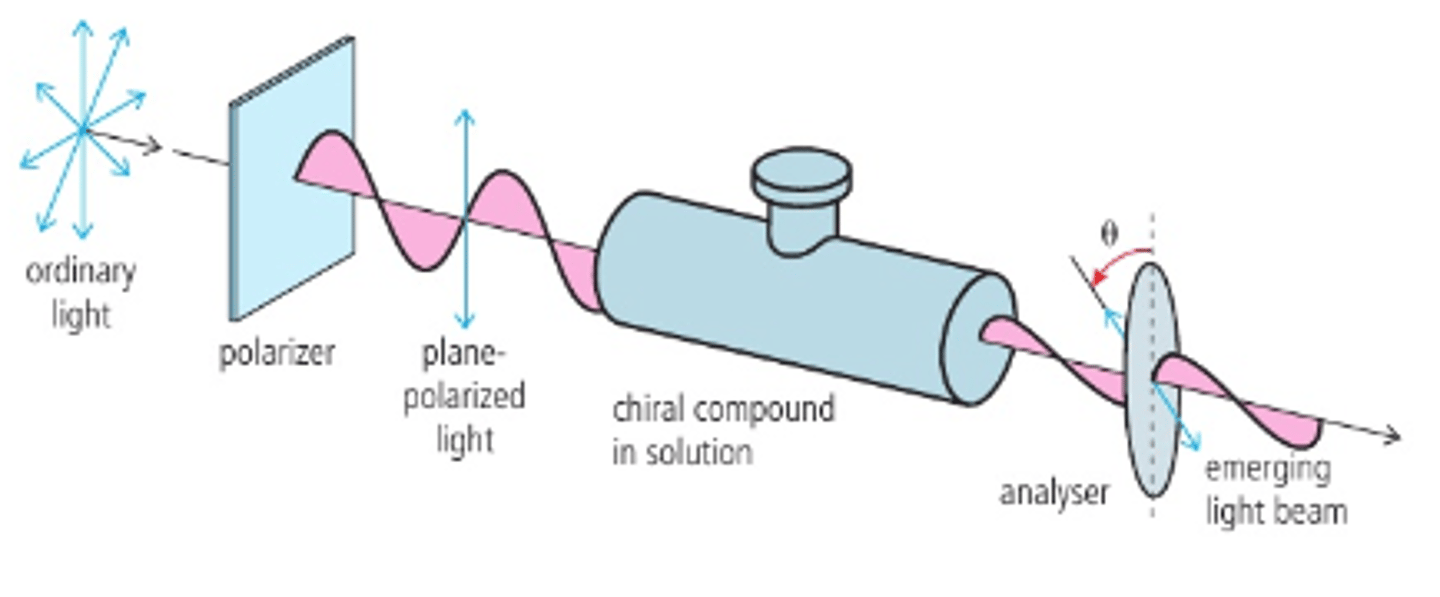
What happens if light is passed through a polarizer?
plane polarised light is passed through a tube containing a solution of the enantiomer
the plane of the plane-polarised light is rotated which then passes through the analyser
the analyser is rotated until light passes through and the the direction of the rotation is measured
Analyser
This can be rotated until the light ppasses through it so the angle of rotation can be deduced.
How do separate solutions of enaniomers, at the same concentration, rotate plane-polarised light?
In equal amounts but in opposite directions.
Optically active
Capable of rotating the plane of polarized light
What is the racemic mixture of a chira compound contain?
Equal concentrations of the two optical isomers.
Optically inactive
A racemic that does not rotate light
What are naturaly occurring chiral molecules?
Optically active, so they exist as only one enantiomer.
When a racemic mixture is reacted with a single enantiomer of another compound
The two components of the mixture, the (+) and (-) enantiomers, react to produce different products which have different chemical properties that can be separated from each other relatively easily.
What happens when you out a racemic mixture through a polarimeter?
opposite rotations cancel ecahother out→ enantiomers are optically inactive
Resolution
A means by which the two enantiomers can be separated from a racemic mixture
Asymmetric synthesis
The process for the manufacture of a single enantiomer using a chiral catalyst.
Qualitative analysis
The detection of the presence but not the quantity of a subtance in a mixture; for example, forbidden substances in an athlete's blood.
Quantitative analysis
The measurement of the quantity of a particular substance in a mixture; for example, the alcohol levels in a driver's breather
Structural analysis
A description of how the atoms are arranged in molecular structures; for example, the determination of the structure of a naturally occuring or artifical product.
Mass spectrometry
Used to determine the relative atomic and molecular mass.
Infrared spectroscopy
Used to identify the bonds in a molecule.
Nuclear magnetic resonance spectroscopy
Used to show the chemical environment of certain isotopes in a molecule
THe ionisation process in the mass spectrometer
This involves an electron from an electron gun hitting the incident species and removing an electron:
X (g) + e → X + (g) + 2e-
The molecular ion, or parent ion
Formed when a molecule losses one electron but otherwise remains unchanged