Metal Alloys
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Thermal, Electrical Conduction, Ductilibility/Malleability of Metal Alloys
Conducts both heat and electricity
Lusters due to inability od electromagnetic radiation to penetrate
Ductile and Malleable due to to non-directional bonding and atomic nuclei being physically moved
Elastic Limit of Metal Alloys
= Proportional limit = yield strength → indicates onset of plastic deformation
Strain hardening increases work hardening and thus raises elastic limit
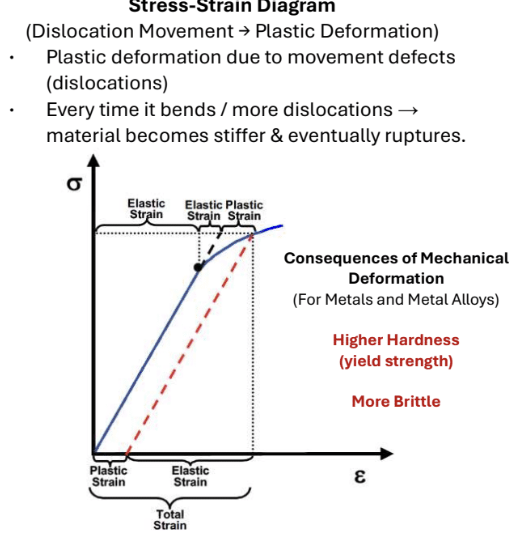
Define Metallic Bonding
Large interatomic forces are created by the sharing of electrons in a delocalized manner to form non-directional bonding
What are the major noble metals used in dentistry
Gold, Platinum an Palladium
Precious metal resistant to tarnish
Silver is not noble, prone to tarnishing
Explain Cast Metal Alloys
Formed into desired shapes by casting in a liquid state or by physically shaping (wrought)
CTE should match or be slightly higher than the material of the veneer porcelain
If CTE is matched appropriately, cooling should not produce fractures
New current ADA Casting Alloy classification
High Noble (HN)
Noble (N)
Predominantly Base Metal (PB)
Base Metal Alloys
High Noble (HN) > must be 40% or more Au and 60% or more total noble metal
TARNISH RESISTANT, corrosion resistant
Noble (N) → must be more or equal to 35% noble
Most common, most common is Ag-Pd (gold substitute) to save money
Predominantly Base Metal (PB) → less than 25% noble
Base Metal Alloys → more than 75% base metal and less than 25% noble
Low cost, oxide formation for bonding porcelain, better for thin casting, high hardness, low ductilibility, low reactivity to oxygen
Passivated with addition of Al, Cr and Ti
Characteristics of HN and N alloys for metal-ceramic prostheses
Can bond to ceramics
CTE matches to ceramic
High melting point for use with low-fusing porcelains
Characteristics of metals
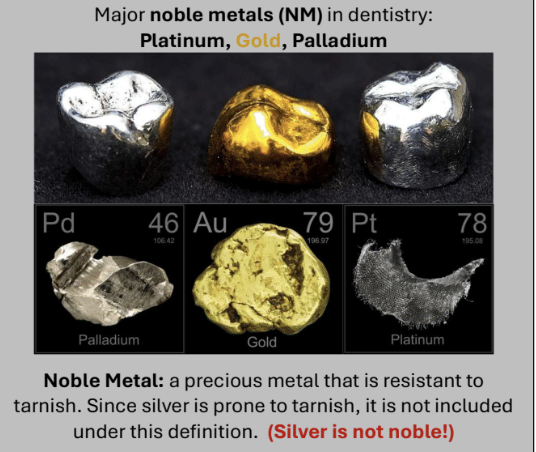
Gold (Au)
Copper (Cu)
Silver (Ag)
Palladium (Pd)
Platinum (Pt)
Zinc (Zn)
Gold (Au): corrosion resistant
Copper (Cu): Higher strength, lower MT, gets tarnished with >16%wt
Responsible for yellow color in yellow gold
Silver (Ag): Higher strength, counteracts reddish tint of copper; not truly a noble metal and tarnishes
Palladium (Pd) and Platinum (Pt): Increases MT, lowers CTE, increases hardness
Responsible for white color of white gold
Zinc (Zn): Oxygen scavenger
Thermal mismatch stresses, what are they and what causes them
Metal and porcelain must have matched LCTE to avoid fractures upon cooling and multiple porcelain applications
No thermal stress issues
Large mismatch can cause fracture, small mismatch compresses porcelain and increases strength
Metal is sandblasted so it mechanically lock into porcelain
PFM vs ceramic preparations are different
Where do errors come from in the fit of indirect cast restorations
The key issue is error propagation due to multiple “hands on” manufacturing steps
Dimensional errors in the impression can worsen as each step is completed
Describe the process of passivation
Oxide layer formed by some elements in base allows which inhibits further oxidation → aluminum, chromium or titanium
Wrought alloys
Rolling or forging an alloy through physical force at levels above yield strength
Facilitated by dislocation movement which increases elastic limit
Displays better properties than cast alloys
Most metal alloys used in dentistry, implants
Define Strain Hardening, cold/hot working, hardening and hardness
Describe the strain-stress curve after work hardening
Strain Hardening: the process of strengthening a metal through deformation due to increasing difficulty for dislocation motion
Done through stamping, drawing, rolling, pounding, bending
Hardening: Increasing the elastic limit of a material
Hardness: Resistance of a material to penetration by an indenter or stylus
Curve: stronger but more brittle, ductility decreased
Hot/Cold- working: work hardening at high or low temp respectively
Re- crystallization
After cold working, the grain of the metals are very disordered, at ½ the MT, re-crystallization occurs and re-precipitates crystals which makes the metal soft and ductile again
Re-crystallization time decreases as the amount of cold working increases
Examples of uses of wrought alloys
Hand instruments: 316L Stainless Steel (S), low carbon, hardened during manufacturing
Clasps for RPDs: made of high noble alloys, clasps must not be too stiff or hard but must be durable
Endo files: SS or NiTi; Nitinol shape memory alloy
Ortho wires and bands: SS, Co-Cr-Ni, NiTi
Composition of Steel, composition of stainless steel and 316L
Steel = Iron + Carbon
Stainless steel = Steel + Chromium
316L = Stainless Steel + 16% Cr + low Chromium
Metal Alloys related to Implants
Almost all implants currently are made of commercially pure titanium (cp-Ti) and special instruments of the same material must be used when placing them as well as cleaning them or they may be scratched and left with iron residue from 316L instruments