Grade 11 Pre-AP Chemistry - Unit 5 Test Review
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
85 Terms
What is Pressure?
force per unit area
measured in pascals (Pa)
How is Pressure Caused?
when molecules collide into a surface
Kinetic Molecular Theory
unless they hit another particle/edge of the container...
-gas particles move in a straight line
-gas particles stay at a constant speed
-gas particles move in the same direction
Properties of Gases
-indefinite shape/volume
-low density
-low attraction between particles
-colourless/odorless
-gas molecules are always moving
STP Conditions
101.3 kPa and 0 degrees Celsius
SATP Conditions
100 kPa and 25 degrees Celsius
conversions for pressure (other units)
101.3 kPa = 760 mm Hg = 760 torr = 14.696 psi = 1 atm (atmosphere)
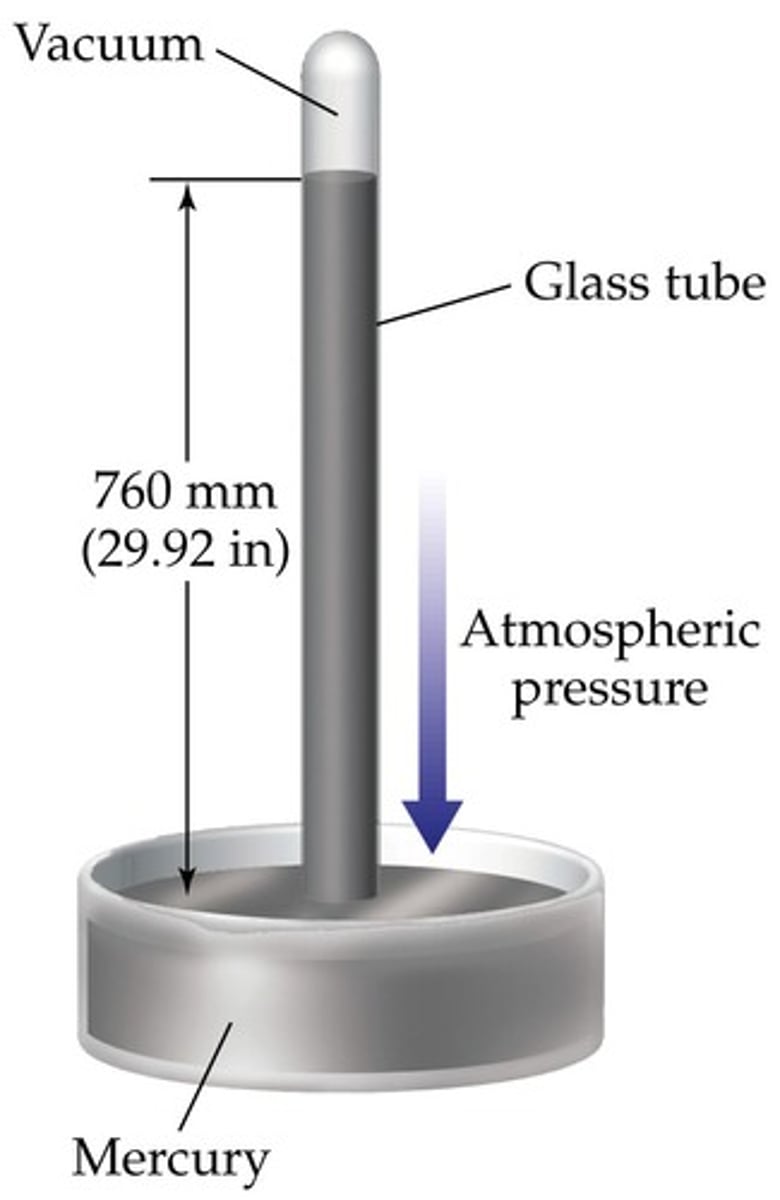
barometer
measures atmospheric P
-open dish of Hg with a tube inverted in it
-as air particles collide with the Hg they exert pressure on it
-since the particles in Hg are already close together, they don't compress, they just move
-the Hg moves into the tube
-the more P, the more mm Hg
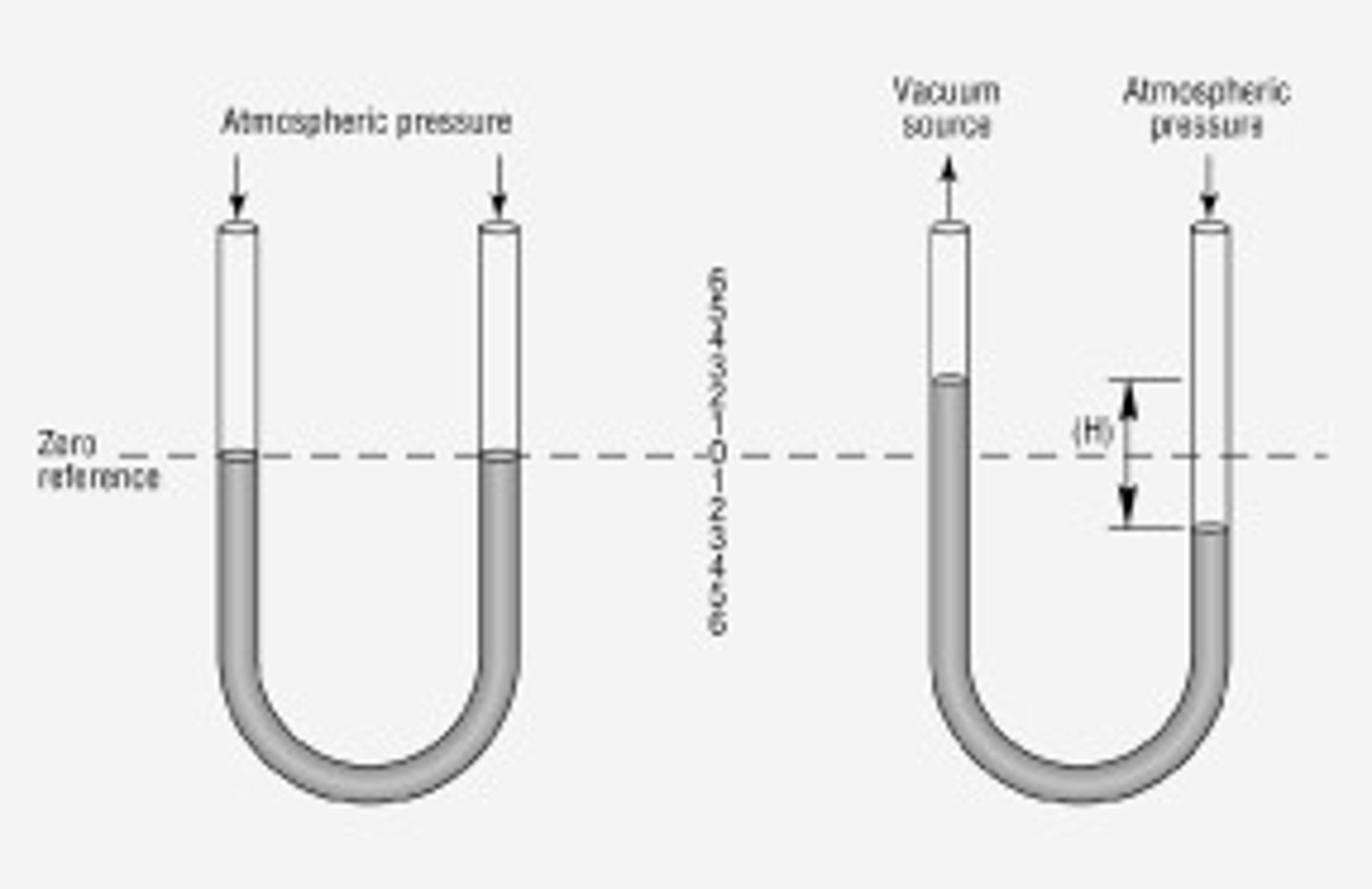
manometer
measures P of other gases
-u-shaped
-Hg in it sinks to the bottom
-gas enters one side; pushes against Hg, causing it to rise on the other side
-P of gas can be found by the difference in height between the two arms of the manometer
Dalton's Law of Partial Pressure
all partial pressures add up to the total pressure
Ptotal = P1 + P2 + P3 ...
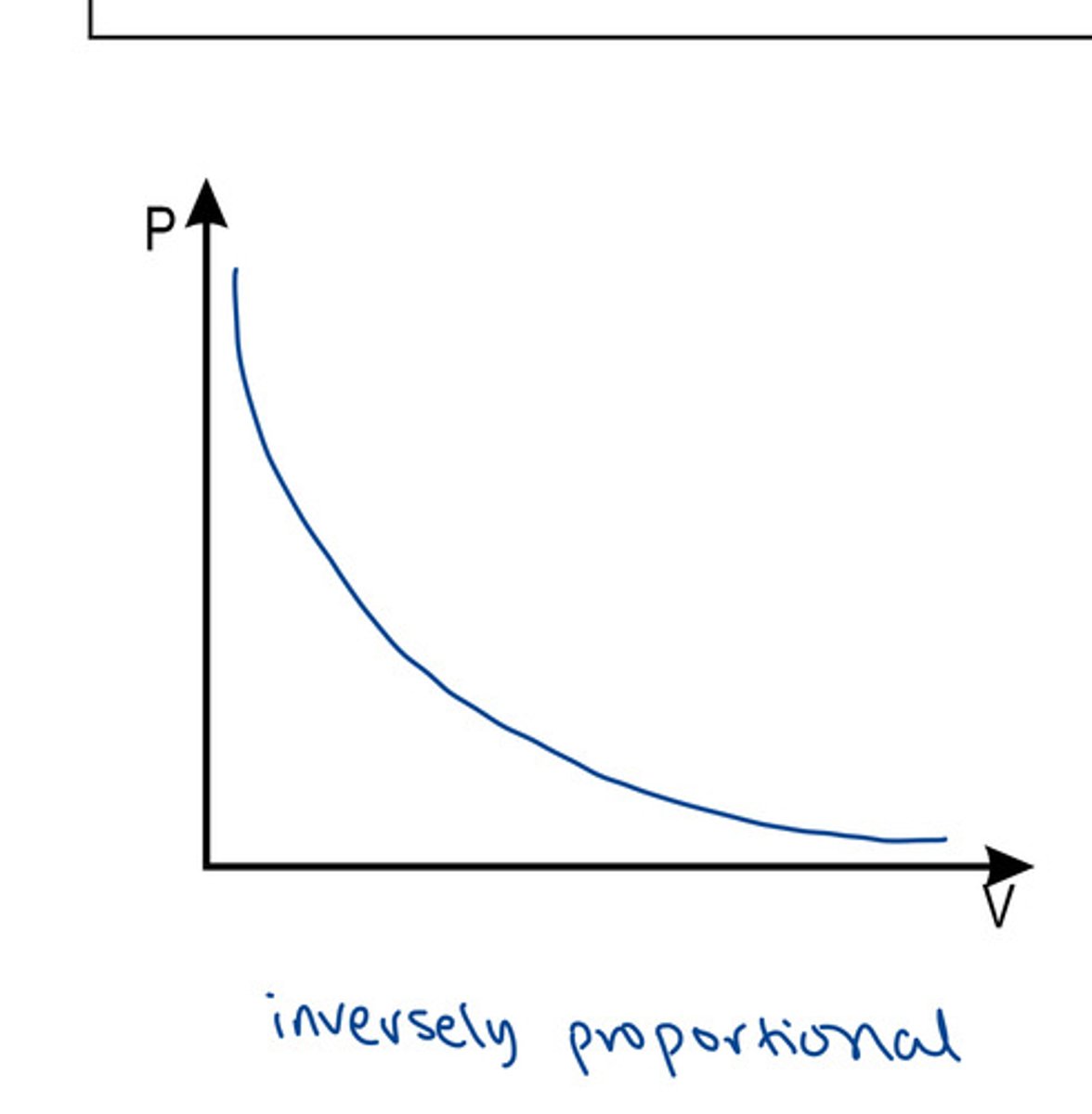
Boyle's Law
when T is constant, P increases as V decreases
P1V1 = P2V2
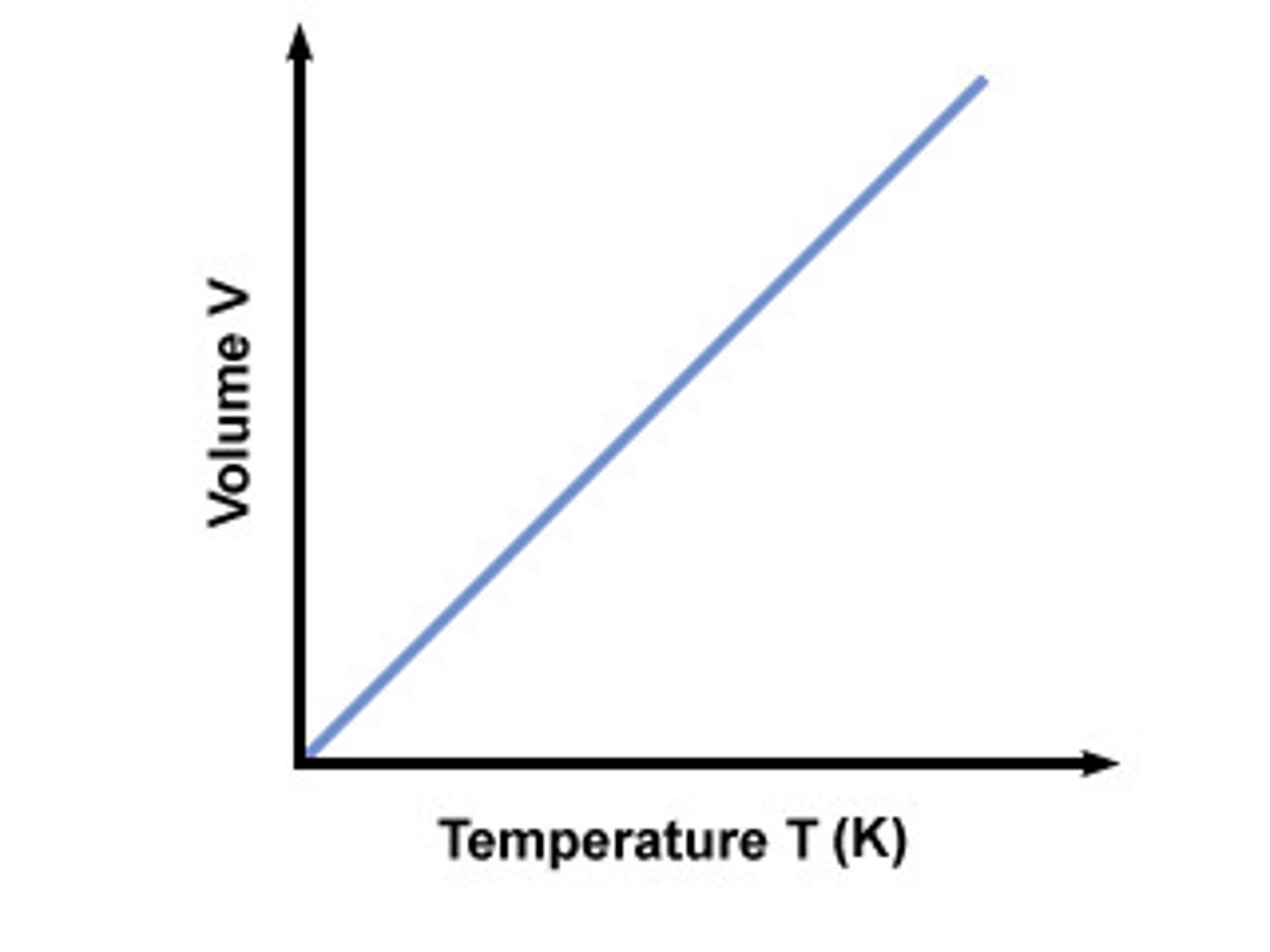
Charles' Law
when P is constant, V increases as T increases
V1 / T1 = V2 / T2
Gay-Lussac's Law
when V is constant, P increases as T increases
P1 / T1 = P2 / T2
What unit are all T's for gases measured in?
KELVIN
Conversion for T from Celsius to Kelvin
T (in Kelvin) = T (in Celsius) + 273.15
Displacement of Water
method to trap + measure P of a gas
-rxn in the flask to produce the gas you want to know the P of
-as gas is generated, it flows through the rubber tubing and bubbles up through the water into the top of the test tube, where it collects
-as more gas collects, the water gets displaced from the test tube
-once all the water is gone, the test tube contains a sample of your gas (WET GAS)
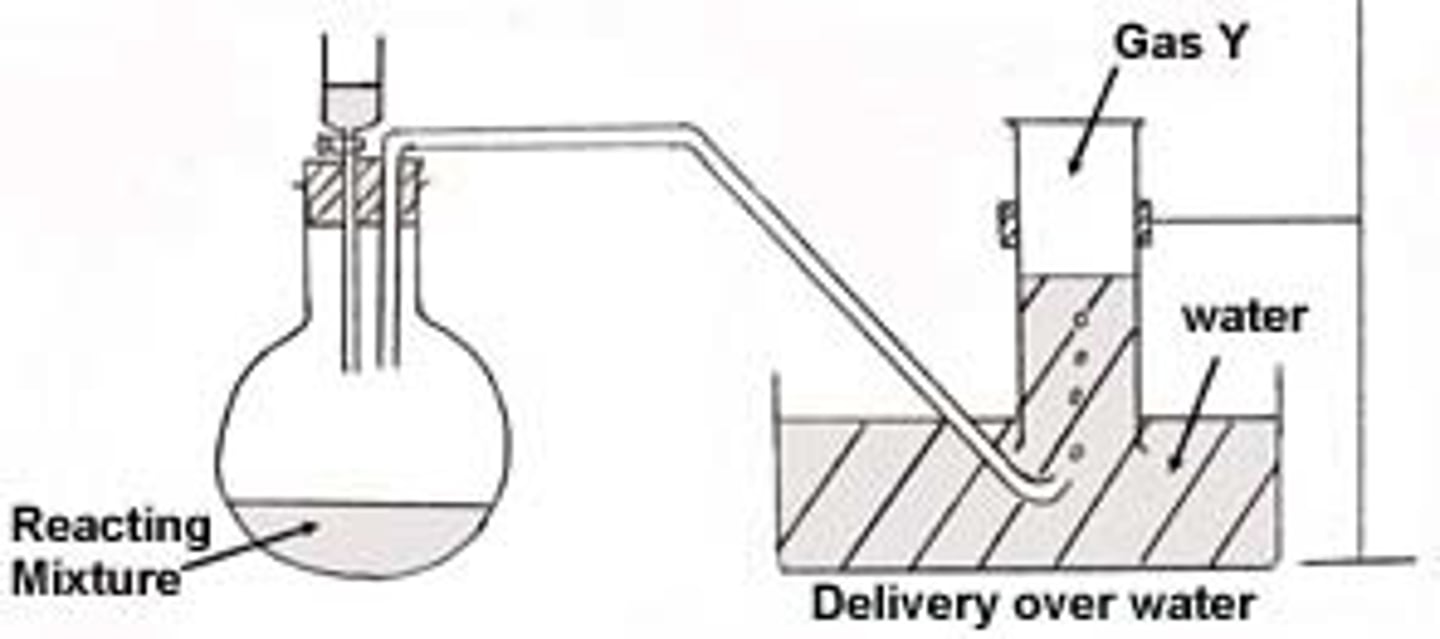
Wet Gas
gas that has water vapour
How do you find the P of the Dry Gas after Displacing Water?
look up the water vapour P at the T you are doing the experiment at, then:
Pdry gas = Ptotal - PH2O
Combined Gas Law
P1V1 / T1 = P2V2 / T2
Greenhouse Effect
natural process in which gases absorb IR emitted from the Earth's surface and radiate it, heating the atmosphere and Earth's surface
Carbon Sequestration
the process of removing CO2 from the atmosphere and storing it
Two Common Types of Carbon Sequestration
biological - plants used to naturally remove CO2
geological - pumping CO2 into depleted reservoirs
4 Layers of the Atmosphere
troposphere, stratosphere, mesosphere, thermosphere
Troposphere (T vs altitude)
T decreases as altitude increases
Stratosphere (T vs altitude)
T increases as altitude increases
-due to higher concentration of ozone
-trapped energy is released to nearby gas molecules
Mesosphere (T vs altitude)
T decreases as altitude increases
Thermosphere
T increases as altitude increases
-due to gas molecules absorbing radiation from Sun
-in the process, they emit visible radiation in the form of auroras
Composition of the Atmosphere
Nitrogen (around 78%)
Oxygen (around 21%)
Argon (around 0.9%)
CO2, water vapour, etc. (rest)
Major GHGs
CO2, CH4 (methane), and water vapour
CO2
GHG
Methane
GHG
Water Vapour
GHG
GHG
gas in the atmosphere that traps IR and radiates it back towards the Earth, heating it past natural standards
Photochemical Smog
smog that contains ground-level ozone, gases, and fine particles
How is Photochemical Smog Produced?
reaction of vehicle + factory emissions + sunlight
Pollutant Gases
sulfur dioxide
nitrogen oxides
carbon monoxide
Sulfur Dioxide
Pollutant gas
SO2 - results from combustion of fossil fuels containing sulfur impurities; reacts to cause acid precipitation
strong choking odour
Nitrogen Oxides
Pollutant gas
e.g. NO - results from vehicles using fossil fuels; contribute to smog/acid precipitation
causes brownish smog
VOCs
volatile organic compounds
-air pollutants
-solid/liquid carbon-containing cpds that vaporize readily
-contribute to formation of smog
-can depress CNS and cause cancer in larger doses
Example of a VOC
gasoline
Ozone (in the stratosphere)
gas that absorbs dangerous UV radiation from the Sun, keeping the planet habitable
Ozone (in the troposphere)
known as ground-level ozone
pollutant - harmful for human health
can cause respiratory issues
How is Ground-Level Ozone Produced?
nitrogen oxides and VOCs react in the presence of sunlight
Particulate Matter
mixture of solid and liquid particles found in the atmosphere
Examples of Particulate Matter
dust, mold, pollen, soot, ash
How is Particulate Matter Produced?
combustion
AQHI
Air Quality Health Index
-scale used to assess the risk of health effects from air pollution
Off-Gassing
release of gases from a substance at room T (e.g. "new car" smell)
Examples of Chemical Pollutants
Methanal - results from off-gassing of paint/cardboard
Carbon Monoxide
Radon - results from radioactive decay of uranium in soil
Methanal
a VOC and chemical pollutant
colourless, flammable gas with a distinctive sharp odour
Sources of Methanal
-paints
-cosmetics
-wallpaper
-cardboard
Carbon Monoxide
(chemical) Pollutant
results from incomplete combustion
difficult to detect (odourless, colourless)
Radon
chemical pollutant
colourless, odourless gas
produced by radioactive decay of uranium in soil and rock
leading cause of lung cancer for non-smokers
Examples of Biological Pollutants
-bacteria
-viruses
-mites
-dust
-pollen
-mold
-humans (shedding of skin cells which are a major component of dust)
Mole Fraction
add up all the moles, then:
the moles of the specific gas / total moles
A mixture of 3 gases has a P of 980 torr. Calculate the partial P of each gas in kPa if there are 3 mol H2, 2 mol CO2, and 6 mol Ne in the container.
Add up the moles to get total moles:
3 + 2 + 6 = 11 mol
Convert the total P from torr to kPa:
980 torr x 101.3 kPa / 760 torr = 130.6236842 kPa
Find the partial P in kPa:
P H2 = (3 mol / 11 mol) x 130.6236842 kPa = 35.62464115 kPa
P CO2 = (2 mol / 11 mol) x 130.6236842 kPa = 23.74993475 kPa
P Ne = (6 mol / 11 mol) x 130.6236842 kPa = 71.24928229 kPa
Factors Affecting the Volume of a Gas
1. Pressure - as P increases, V decreases
2. Temperature - as T increases, V increases
3. Amount of Particles (moles or n) - as n increases, V increases
Ideal Gas Equation
PV = nRT
R in Ideal Gas Equation
8.314 (kPa)(L) / (mol)(K)
What is an Ideal Gas?
a hypothetical gas in which the particles don't interact at all, all collisions are completely elastic, and all gas laws are followed exactly with V dropping to 0 as T approaches absolute 0
Avogadro's Law
as n increases, V increases
n1 / V1 = n2 / V2
Molar Volume of a Gas at STP
22.4 L
Density Formula
D = P x MM / RT
OR
D = mP / nRT
How do Particles Behave at Absolute 0?
no movement, no thermal energy, V should drop to 0 as T approaches absolute 0
Ideal Gas vs Real Gas
actual gases ARE NOT ideal gases, but they will act like one as long as conditions are not extreme
Differences Between Solids, Liquids and Gases with Kinetic Molecular Theory
Vibrational motion: gases, Liquids and Solids
Rotational motion: gases and Liquids
Translational motion: gases and liquids
Vibrational Motion
vibration of particles
Rotational Motion
a particle spinning in place
Translational Motion
the movement of a particle in straight lines
How is Pressure Affected as Altitude Changes?
higher altitude - lower pressure
lower altitude - higher pressure
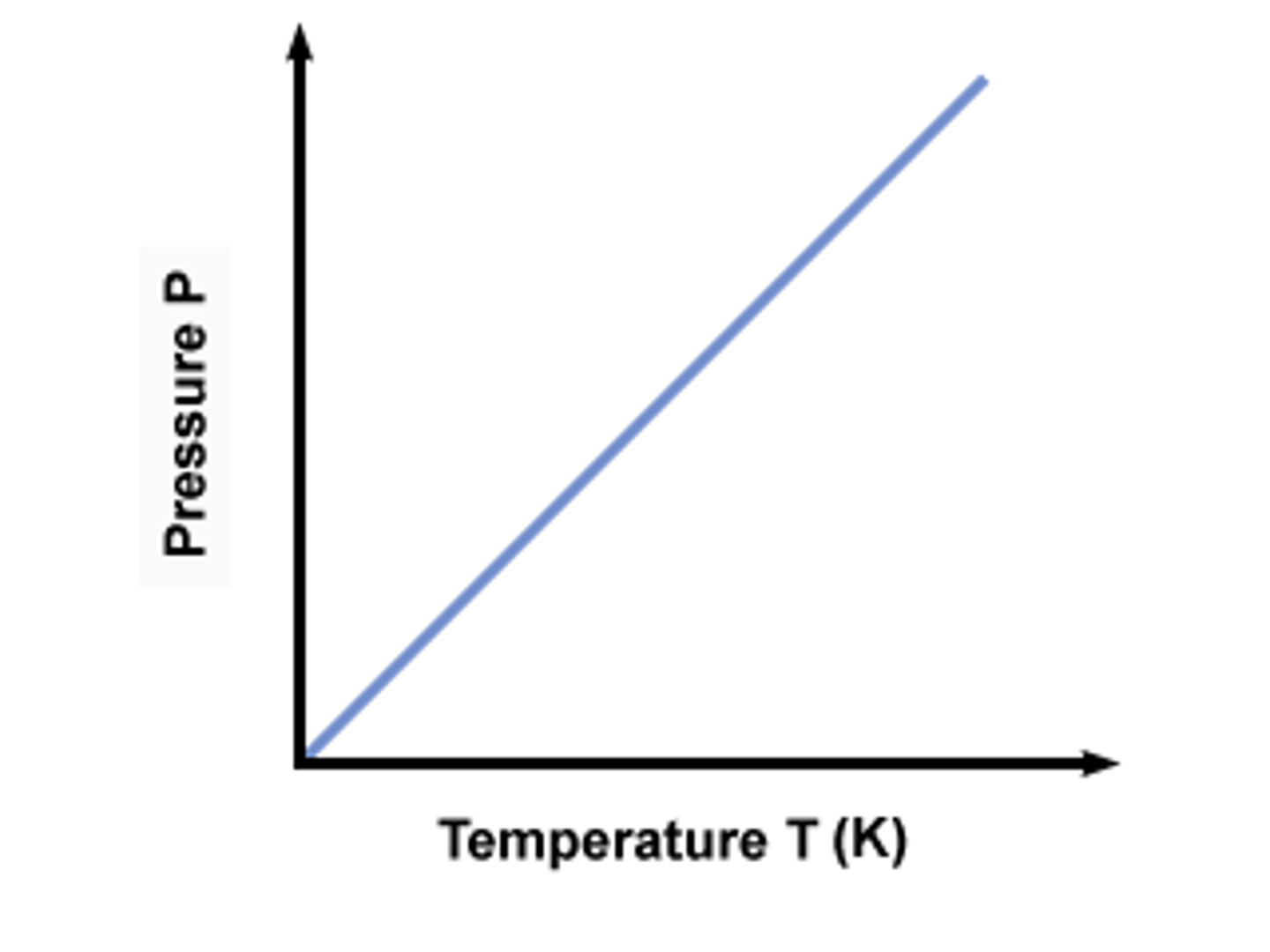
What does the graph between P and T look like?
What does the graph between V and T look like?
What does the graph between V and P look like?
N/m^2 and Pa
1 N/m^2 = 1 Pa
Why doesn't it matter what gases are added to a container?
because the particles are so far apart, there is minimal interactions between them, therefore, the gases will behave separately (as long as no chemical reaction occurs)
2 things needed to consider when collecting a gas over water
1. cannot use a gas that dissolves in water
2. the gas will be a 'wet gas' (have to subtract the P of the water vapour in the gas to get the P of the 'dry gas') - using chart
What does it mean when you see the words 'collected over water' in the question?
you need to subtract the water vapour P using Dalton's Law of Partial Pressures
What substance gives smog its reddish-brown colour?
nitrogen dioxide
Nitrogen
most abundant gas in the atmosphere
important in all biological systems
Where is most of the ozone in the atmosphere concentrated?
stratosphere
Where is most of the water vapour in the atmosphere concentrated?
troposphere
What are the 2 requirements of a mixture of gases for Daltons Law of Partial Pressures to apply to it?
1. the gases must not react
2. the units of P must be the same
how to solve for the mass of a gas using ideal gas law equation
PV = nRT
since n = mass / Molar Mass,
PV = (m / MM) x RT
m / MM = PV / RT
m = PVMM / RT
how to solve for the molar mass of a gas using the ideal gas law equation
PV = nRT
PV = (m / MM) x RT
m / MM = PV / RT
PVMM = PTm
MM = RTm / PV
how to solve for the density of a gas using the ideal gas law equation
PV = nRT
P(m / D) = nRT
m / D = nRT / P
DnRT = mP
D = mP / nRT