GCSE Physics P3: Particle Model of Matter
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
density (2)
- mass per unit volume
density = mass/volume
solids, liquids and gases
- solids and liquids have similar densities as the space between particles however liquids have a lower density than solids
- gases have far lower density as the spacing between atoms increase x10 as particels have a lot of energy to move, so volume increases greatly so density decreases molecules in a gas are in constant random position
change of state
- mass is conserved
- physical changes are revsersible but not chemical changes as the materia retains its original properties when reversed
solid to gas
gas to solid
sublimation (dry ice/solid co2)
desublimation
initial energy
sum of kinetic and potential energies of all the particles in a system
specific heat capacity
amount of energy required to raise the temperature of 1kg of substance by 1 degrees celsius
change in thermal energy equation
mass x specific heat capacity x temp change
Specific Latent Heat of Fusion
The energy required to change the state of 1 kg of a substance from solid to liquid at a constant temperature.
It is released during freezing and taken in when melting
Specific Latent Heat of Vaporisation
The energy required to change the state of 1 kg of a substance from liquid to gas at a constant temperature.
It is released during condensation and taken in during boiling
energy for a change of state equation
mas x specific latent heat
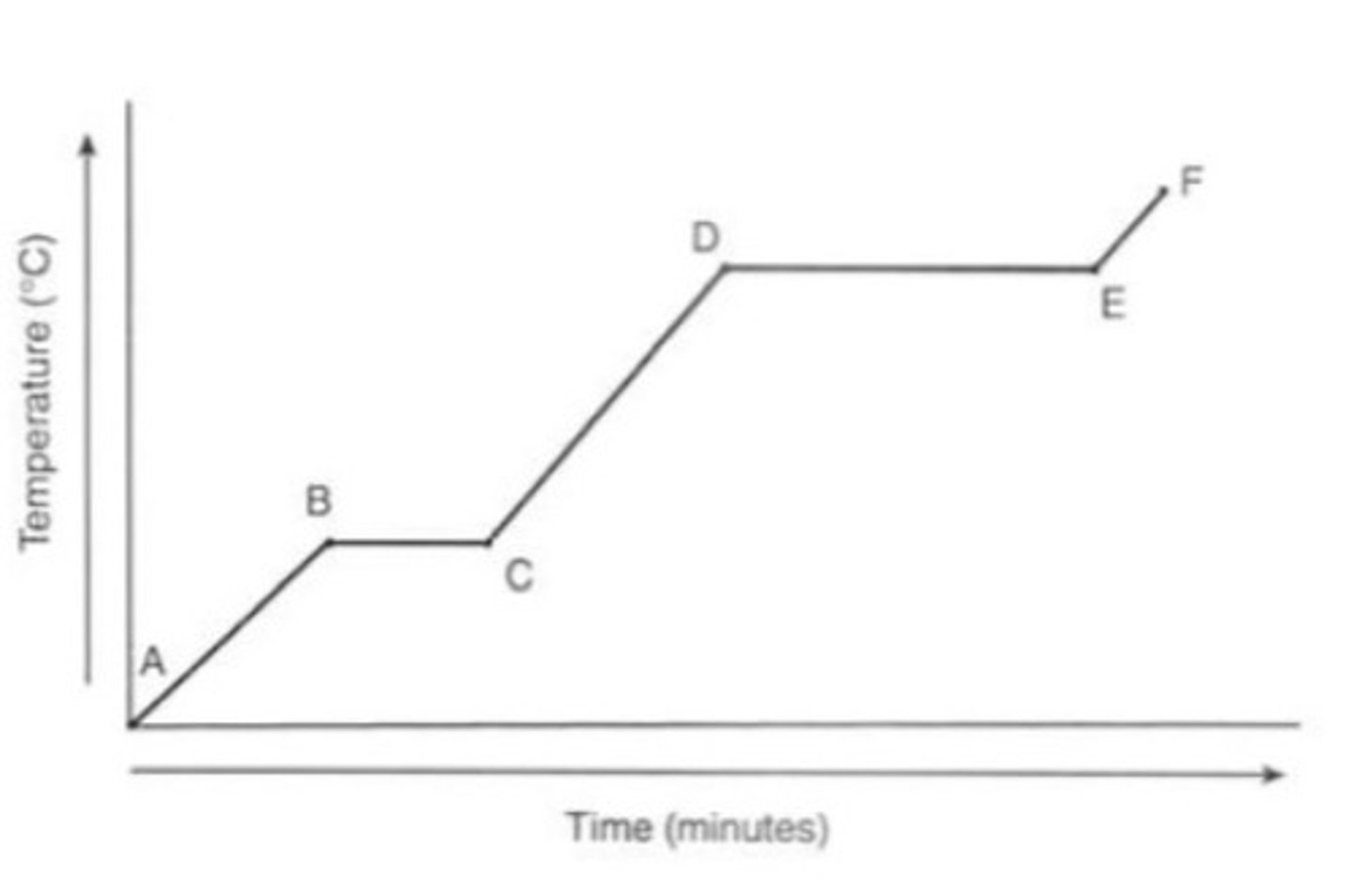
graph showing temp of ice (6)
- At A it is a solid
- At B reaches 0 degrees
- From B to C there is no temp change because the energy is used through melting
- From C to D it is in a liquid state
- From D to E the water is boiling and it takes longer because evaporation takes more energy
- From E to F the gas is heating
Pressure in a gas
Gas particles collide with the walls of the container
Each collision exerts a force
The force is at right angles to the surface
The pressure is the total force applied over the area of the container in a given time
P=F/A
Pressure in a gas, increasing the temperature
Increasing the temperature increases the speed of gas molecules
There are more frequent collisions with the walls of the container
Each collision exerts a larger force
The total force across the area of the container in a given time increases
So the pressure increases
Pressure in a gas, decreasing the volume TEMPERATURE CONSTANT
There are more frequent collisions with the walls of the container
The total force across the area of the container in a given time increases
So the pressure increases;
work done equation
work done = force x distance = force/area x (area x distance) = pressure x volume
Model Answer: Work done on a gas TEMPERATURE CHANGES
Work is done on the gas when decreasing its volume.
This increases the internal energy of a gas
And so the temperature increases.
(the opposite is true if the volume increases)
Boyle's Law
Pressure and volume in a gas are inversely proportional at a constant temperature;
Pressure and volume in a gas are inversely proportional at a constant temperature;
- the particles collide with the wall which is moving inward
- so the particles gain momentum as the rebound velocity is greater than the approaching velocity
- so as the particle has a greater velocity