Freshwater Ecology Lab Exam
1/129
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
130 Terms
Microcystis taxonomy (Kingdom, Phylum, Order, Genus)
Kingdom: Monera
Phylum: Cyanophyta (cyanobacteria
Order: Chroococcales
Genus: Microcystis
Volvox taxonomy (Kingdom, Phylum, Class, Order, Genus)
Kingdom: Protista
Phylum: Chlorophyta (Green algae)
Class: Chlorophyceae
Order: Volvocales
Genus: Volvox
Diatoms Taxonomy (Kingdom, Phylum, Class, Orders)
Kingdom: Protista
Phylum: Bacillariophyta (diatoms)
Class: Bacillariophyceae
Orders: Centrales and Pennales
Euglena taxonomy (kingdom, phylum, genus)
Kingdom: Protista
Phylum: Euglenophyta
Genus: Euglena
Paramecium Taxonomy (Kingdom, Phylum, Genus)
Kingdom: Protista
Phylum: Ciliophora
Genus: paramecium
Rotifera taxonomy (kingdom and phylum)
Kingdom: animalia
Phylum: rotifera
Daphnia taxonomy (Kingdom, Phylum, Subphylum, Class, Genus)
Kingdom: animalia
Phylum Arthrophyta
Subphylum: Crustacea
Class: Branchiopoda
Genus: Daphnia
Stentor taxonomy (Kingdom, Phylum, Genus)
Kingdom: Protista
Phylum: Ciliophora
Genus: Stentor
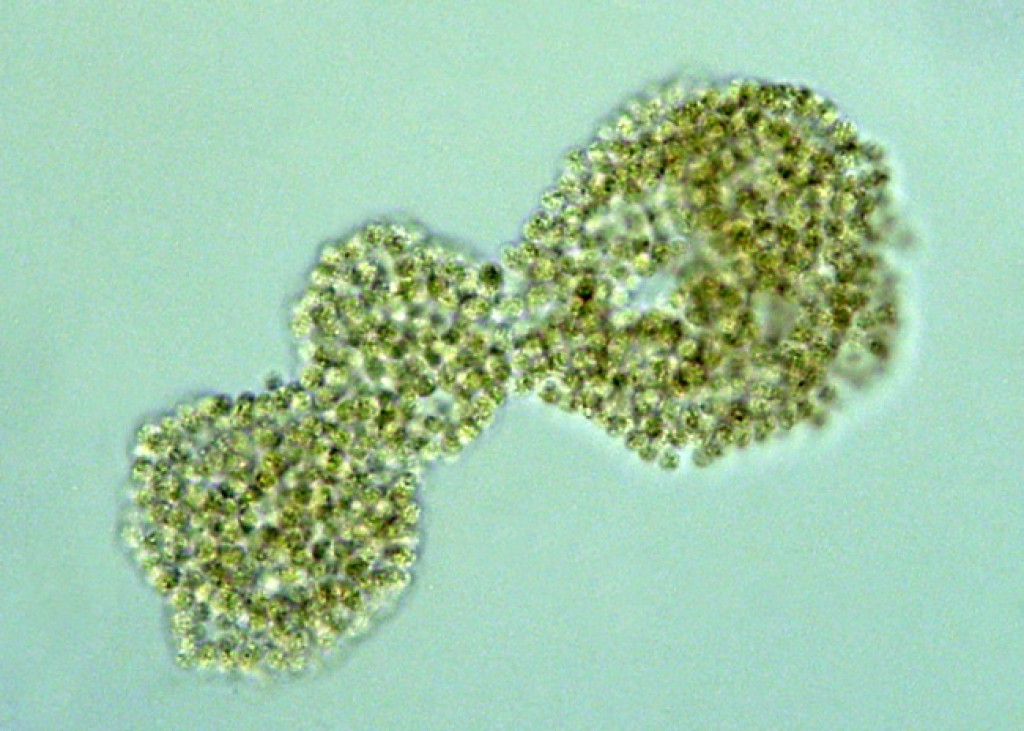
identify the organism
microcystis
identify the organism
volvox

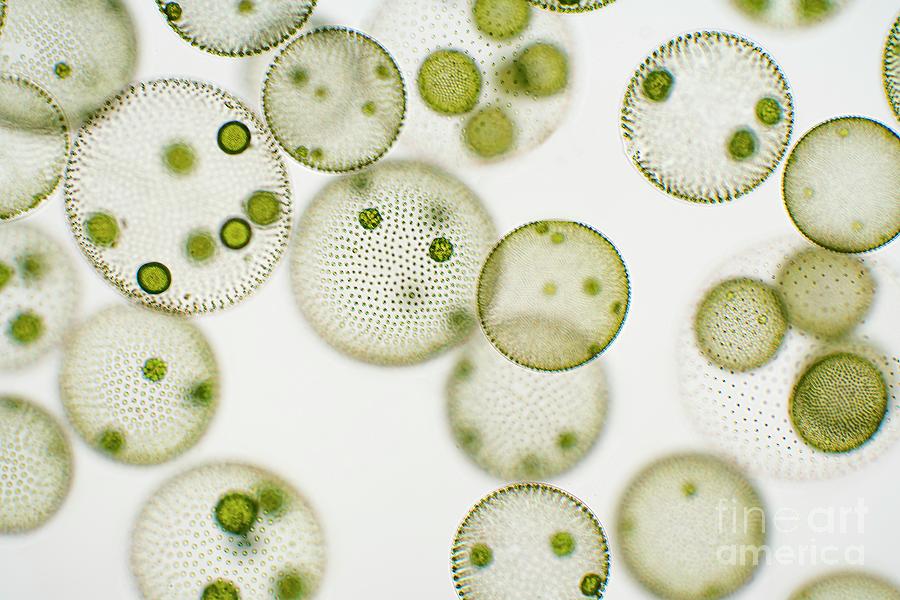
identify the organism
diatoms
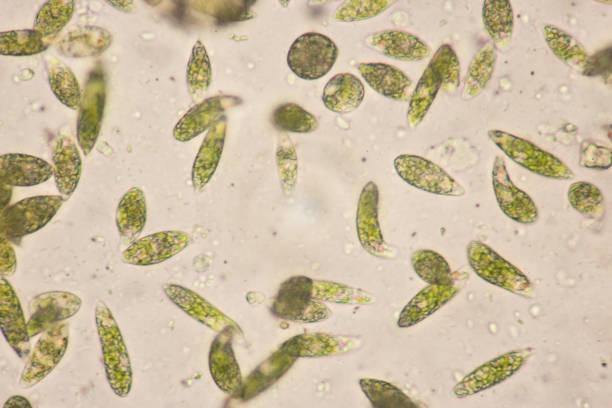
identify the organism
euglena
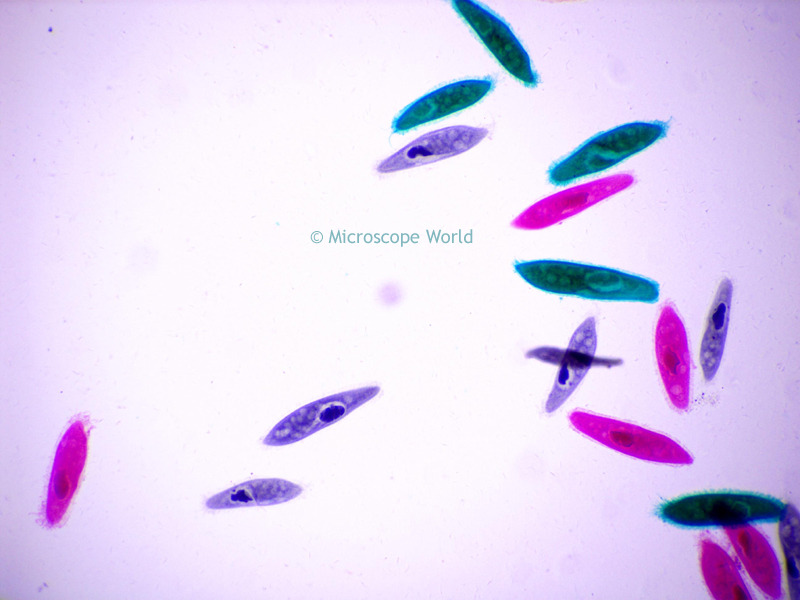
identify the organism
paramecium
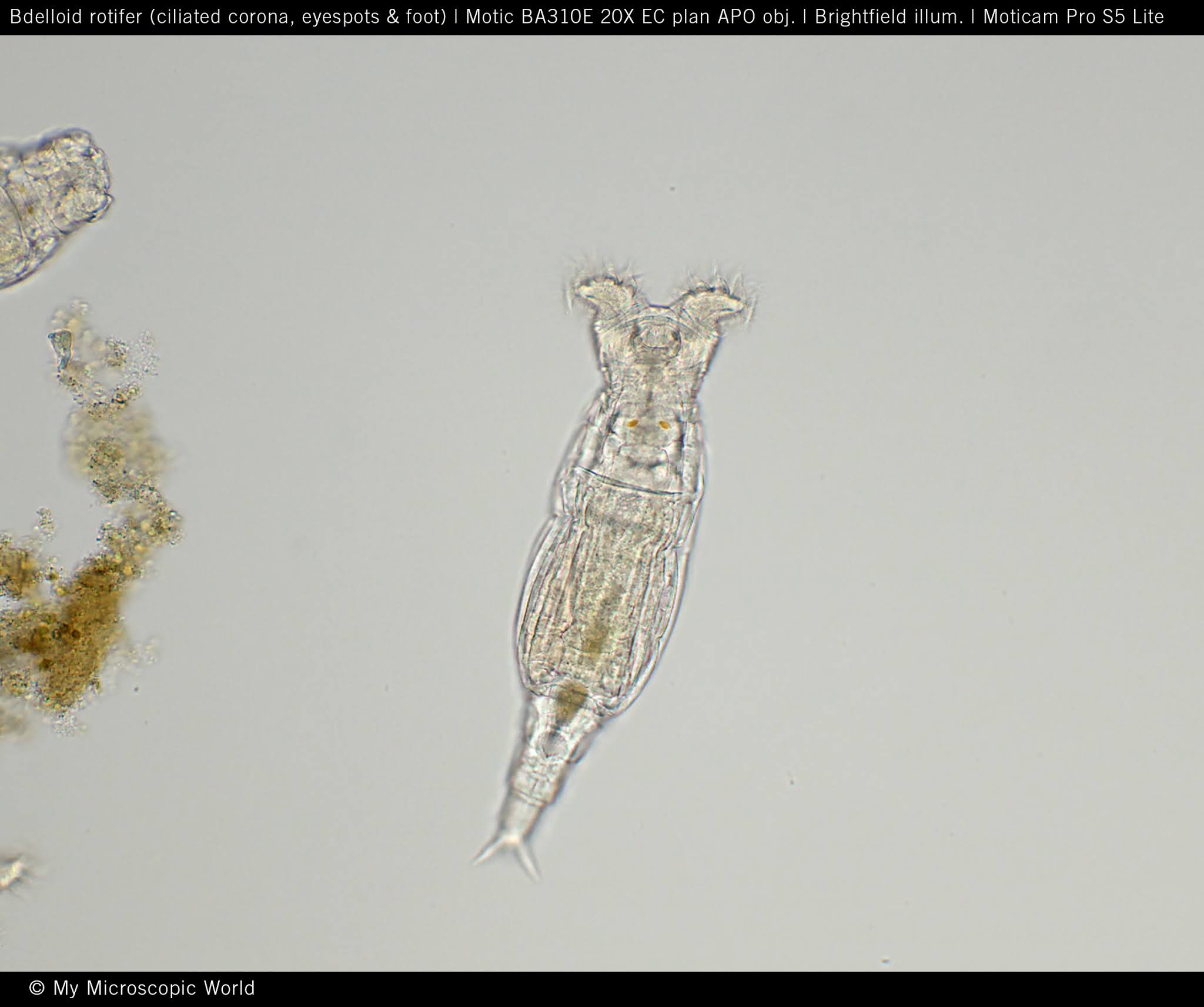
identify the organism
rotifer
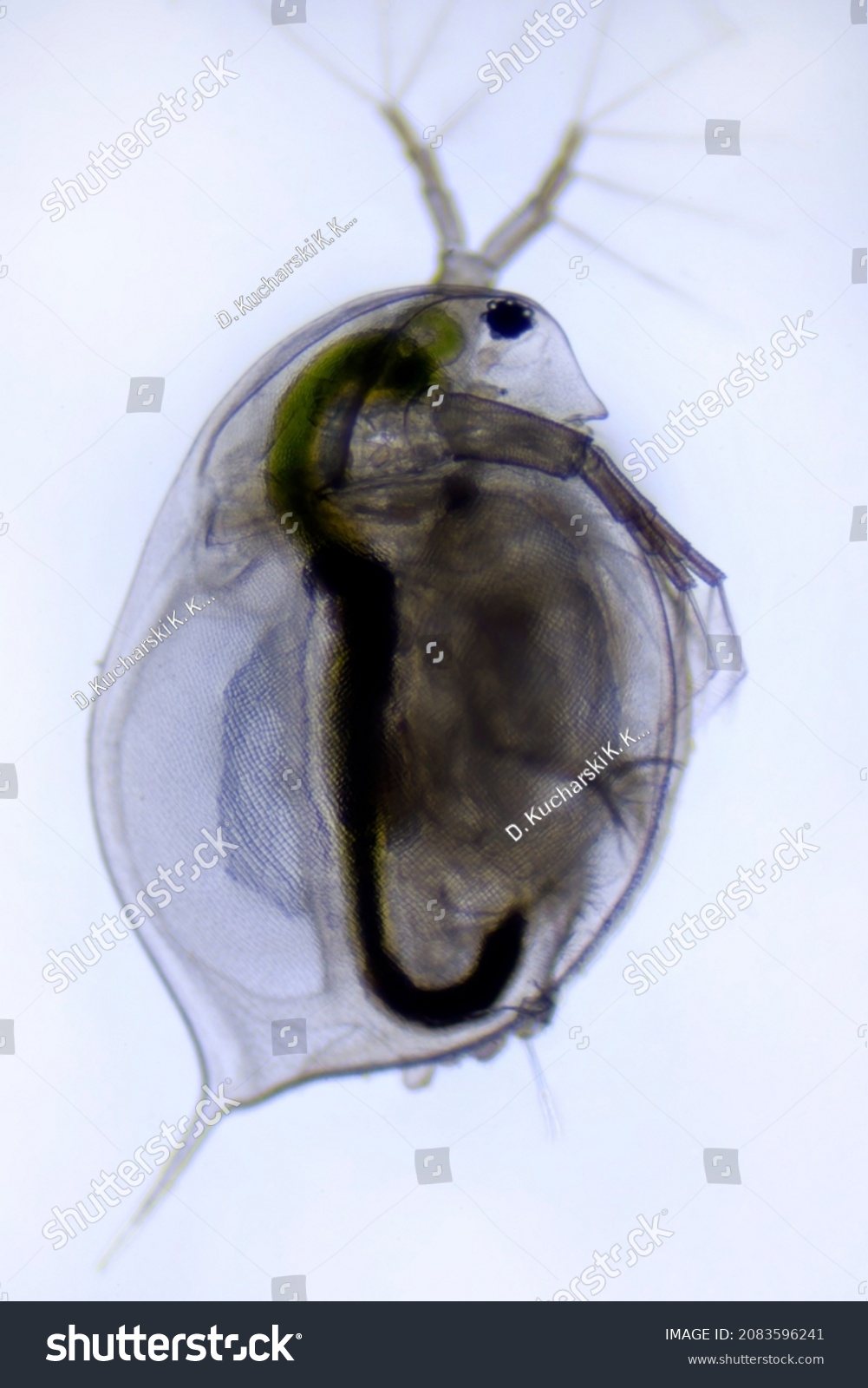
identify the organism
daphnia
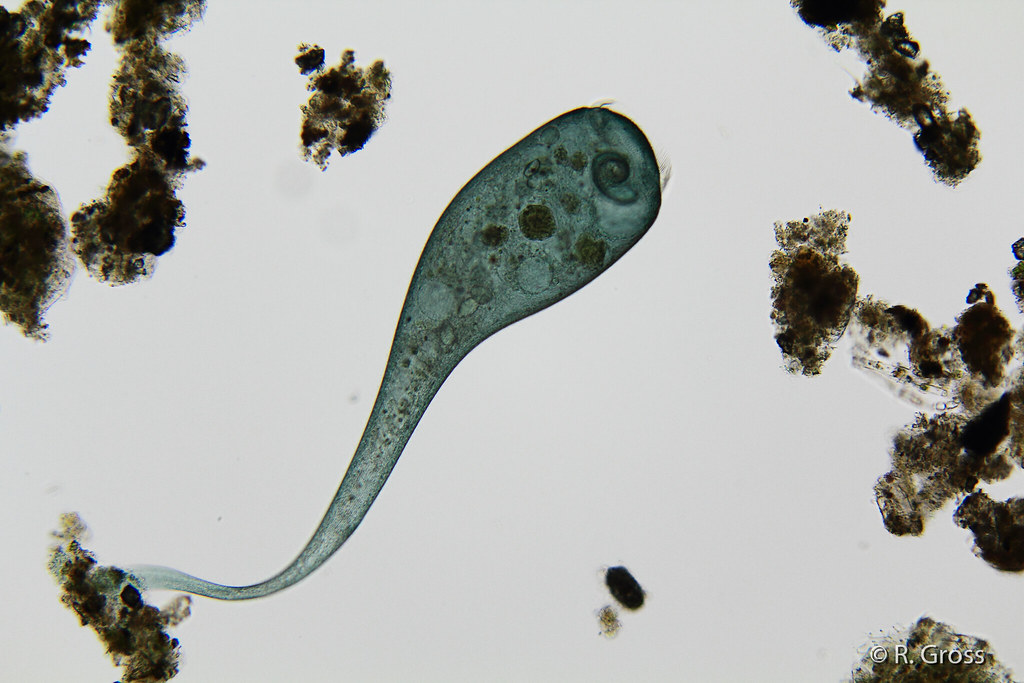
identify the organism
stentor
what was the name of the site we sampled at for our field trip
sturgeon creek
hold time and purpose
samples must be collected and brought to the lab within 48 hours
the chemistry of the sample will change over time if not analyzed right away
ice helps slow biological and chemical reactions
two types of quality control samples
trip blank and field blank
trip blanks
sample of lab water is simply
transported into the field and back, then analyzedresults indicate if the bottles were cleaned properly in the lab
field blanks
use lab water, but treat it as an regular sample
sample its poured though all equipment and sample bottles first and will receive preservative if used for other samples
determines if field techniques were done properly, if the preservative was contaminated, and if the air contaminated samples
Grab samples
into bottle: samples collected directly into bottles
the bottle is inserted into the water open end down and turned horizontally to the current. Then its is turned upright while still in the water and brought up out of the water to be sealed
ways to obtain larger and deeper grab samples
Kemmerer, Van Dorn and Niskin samplers
lugol’s solution
preserves the samples when they can not be looked at quickly
what is a phytoplankton trawl net
used to get non-quantitative samples of phytoplankton
advantages of phytoplankton trawl nets
obtain a large sample of biomass in a
short period of timecan see a good range of the organisms present
disadvantages of phytoplankton trawl nets
loss of small algae
contamination with larger numbers of
zooplankton, which selectively consume the algaeparticulate inorganic and organic debris that may have an associated bacterial population collected
concentrated biomass leads to change in water quality during transport
peristaltic pump
used to get large grab samples
creates a vacuum to obtain sample
ways to obtain depth integrated samples
iron bottles
sampling tubes
obtaining multiple samples at discrete depths and mixing them together to get a final sample using Kemmerer or Van Dorn samplers
how to avoid contamination of zooplankton in a phytoplankton trawl net
you can use a larger mesh net inside a smaller mesh net
Schindler-Patalas Sampler (qualitative zooplankton sampler)
water flows free through the clear box until desired depth is reach
then doors on box are closed and the box is pull up out of the water
water exits the box through the small mesh net, collecting zooplankton in the small bottle at the bottom
why take macrophyte samples
contribute significantly to the productivity of freshwater systems
provide substrates/habitat for other organisms
influence thermal stratification by reducing mixing
influence the oxygen content of the water because of their high photosynthetic rates
work to stabilize bottom sediments
excrete organic compounds that inhibit growth of phytoplankton and bacteria
ways to collect qualitative macrophyte samples
by hand using wader
double sided rakes
Ekman dredge
Louisiana box sampler
Osborne frame sampler
determining macrophyte biomass
(dry) weight of plant material taken from a unit of bottom area at a given
timeconsidered standing crop if bellow ground portions not included
considered biomass if bellow ground portions included
Ekman dredge
size used depends on organism density and sediment softness
doors swing open to let water in as it is lowered into the water
once at the bottom pull up one meter and let the dredge free fall so it penetrates the sediment
then the messenger is released to close the jaws and retrieve the sample
contents are then sifted with bucket with a sieve at the bottom
why take benthic macroinvertebrate samples
diversity is generally high in stable environments with a balanced distribution of species
Physical/chemical stresses can result in a shift in the community makeup, causing sensitive organisms to disappear, while tolerant species become disproportionately dominant
ways to obtain benthic macroinvertebrate samples
Ekman dredge
kick sampler
tow nets attached to boats
Ponar grab
Peterson grab
sediment corers
collect sediment samples for chemical analysis
assess changes in sediment composition over time
sediments are laid down annual like tree rings, providing a historical record
List of water quality parameters that need to be measured
temperature
dissolved oxygen
pH
conductivity
light
stenothermic
adapted to a narrow temperature range
eurythermic
adapted to a wide range of temperatures
thermocline
thin layer in the water column where temperature
changes more rapidly with depth than the layers above or below it
why measure temperature
some organisms are stenothermic or eurythermic
impacts the amount of gases dissolved in water (ex: colder = more DO)
impacts rates of chemical reactions
presence of thermocline prevents mixing of nutrients between hypolimnion and epilimnion
epilimnion vs hypolimnion
epi - above thermocline
hypo - below thermocline
how is temperature measured
thermistor thermometer
The device is made of metals whose electrical resistance
depends on temperature. A change in resistance to an electrical current
indicates a change in temperaturecan be placed on a long cable so temperature can be read at different depths
why measure dissolved oxygen
required for all animals in the water column, the benthos organisms in the substrate, and plants/phytoplankton
many organism sensitive to dramatic DO changes
fluctuates diurnally - added by photosynthesis during the day and consumed by respiration at night
depends on depth, organic matter, and sediment type
depletes in the winter
how is dissolved oxygen measured
electrometric technique (yellow springs instrument)
based on the rate of diffusion of molecular oxygen across a membrane
measure temperature as well
probe can be attached to a long cable to take reading a different depths
how to measure pH
based on the use of a pH sensitive glass electrode and a
reference electrode (e.g. a silver chloride electrode) and a temperature
element to provide a temperature signal to the pH analyzer (as pH is
temperature dependent)the difference in the potentials of the glass and reference electrodes when placed in a solution provide a millivolt signal proportional to the pH
conductivity
The electrical conductivity of water is the ability of water to conduct an electrical current, which is dependent on the concentration of ions it contains
The greater the ionic concentration (dissolved salts etc.), the greater the conductivity
why measure conductivity
greater concentration of dissolved ions = greater productivity
provides a rough indication of the amount of total dissolved solids within the water
how to measure conductivity
temperature dependent (increases 2-3 percent per increases degree of temp)
measurement is made by a probe that uses two electrodes
placed 1 cm apart, so the results are often reported as μmho/cm
why measure light
provides energy for photosynthesis
heats the water
helps organisms see
different wave lengths of light penetrate water at different depths
what does light penetration depend on
angle the light is hitting the water at (the straighter the angle, the more is reflected)
the types and sizes of waves
material that may be on the surface of the water
extinction of light with depth is due to
the water itself
suspended particles (such as clay or phytoplankton)
dissolved organic substances such as humic acids that colour the water
photic zone
depth to which 1% of the PAR penetrates the water
light compensation points
When an organism is at their light compensation point their production rate
equals their respiration rateif they fall below this level in the water column they
will survive only until they use up their energy stores
surface inhibition
organisms can be damaged at the direct surface due to high light intensity
phytoplankton tend to be most dense at some point below the surface of the water
how to measure light
photometer ( can be used to determine the depth of the photic zone by taking multiple readings at different depths
secchi disk - The disk, tied to a calibrated line, is lowered
into the water and the depth where it disappears is
recorded. It is then lowered further another m and
raised. The depth that it reappears to the observer
is also recorded. The average of the first and
second readings is the Secchi Disk Depth
why sample/ study plankton
contribute oxygen to the atmosphere
sensitive to various pollutants and nutrient inputs = changes occur in plankton before they are visible in larger organisms = bioindicators
healthy ecosystem = diverse plankton
unhealthy = little diversity and many undesirable plankton
food source for larger organisms
Characteristics of genus Microcystis
Unicellular but form globular colonies covered with a layer of mucilage
for large blooms
produce microcystins - hepatotoxins
toxins releases upon cell death or lysis
general characteristics of all Cyanophyta
prokaryotic (lack organelles but have granular inclusions)
photosynthetic pigments associated with thylakoid membranes
thin cell wall made of peptidoglycan and lipopolysaccharide
optimal growth at high temperature
tolerate low light and N:P ratios
reproduction is vegetative - cell division and spore formation
colonial or unicellular forms
different adaptations Cyanobacteria may have
gas vacuoles
heterocysts
akinetes
mucilage layer
colony formation
production of toxins
gas vacuoles
allow cyanobacteria to change their vertical position in the water column
float down to obtain nutrients
float up to obtain light for photosynthesis
heterocysts
specialized cells that fix nitrogen
have special cell wall layers that prevent oxygen from diffusing into the cell
advantage in low nitrogen environments
akinetes
thick walled spore-like cells
able to survive harsh conditions
ensure that an organisms genetic information is passed on
general characteristics of Chlorophyta class Chlorophyceae
green color due to dominance of chlorophyll a and b
unicellular, colonial, and filamentous
microscopic and macroscopic
motile and non-motile
starch reserves stored in plastids
eukaryotic
planktonic and benthic
rarely form blooms
morphology of diatoms
yellow-brown color
thick silica cell walls
non-flagellate
unicellular, colonial, or chains
2 shapes: centric (discoid or cylindrical with radial symmetry) and pennate (elongate with bilateral symmetry)
diatoms habitat preferences
standing and running water
planktonic, benthic, epiphytic, epizoic
tolerant to low light and and low temperature
form blooms in fall and spring
well preserved in sediment
function of silica cell wall in diatoms
aka frustule
requires less energy to make than the other types of algal cell walls
gives them a head start in spring
disadvantage of silica cell wall in diatoms
the frustules are heavy and cause the diatoms to sink to the bottom of the water column
require turbulence to to remain suspended
ways diatom colonies form
mucilage pads - occur at the ends of cells, allowing them to join
interlinking spines where where chains of cells are joined valve to valve
gelatinous stalks - used to attach to the substrate and join small groups of cells together
Euglenophyta morphological characteristics
unicellular with flagella
anterior depression where flagella immerge
have an eyespot
surface coat/pellicle is flexible
paramylon storage reserves
Euglenophyta feeding preferences
1/3 are photosynthetic - elongate, spindle shaped, with multiple chloroplasts
the rest are heterotrophic or phagotrophic
genus euglena are facultative heterotrophs
prefer environments with abundant decaying matter (shallow lakes, wetlands, farm ponds)
Rotifera characteristics
unsegmented
pseudocoelomate
ciliated apical region known as the corona
a muscular pharynx known as the mastax
cilia are used for filter feeding and locomotion
critical role in microbial nutrient loop
daphnia and other branchiopod characteristics
leaf-like thoracic legs (phyllopods)
eat algae and detritus and often consume the bacteria on benthic or suspended organic matter
what were we trying to determine in the chlorophyll experiment
Do the algae respond to changes in nutrient levels (N and P) of their growth medium?
why use chlorophyll a to determine phytoplankton growth
all algae contain chlorophyll a because it is required to capture energy from the sun to be used in photosynthesis
makes photosynthesis possible by passing its energized electrons onto molecules that manufacture sugars
therefore chlorophyll a can be used a bioindicator for algae
chlorophyll accounts for what percent of algae dry weight
0.9 – 3.9%
what happens when chlorophyll a is exposed to light
can degrade to phaeophytin
why does conversion of chlorophyll a to phaeophytin cause inaccurate results
phaeophytin absorbs light in the same wavelength as chlorophyll a
can result in an artificially high concentration of chlorophyll a reported
what causes high phaeophytin
many dead phytoplankton
or they are nearing senescence
indicates poor physiological condition (health) of phytoplankton
healthy ratio of chlorophyll a to phaeophytin
A ratio of 1.7 chlorophyll a to phaeophytin
indicates little degradation occured
unhealthy ratio of chlorophyll a to phaeophytin
ratio of 1 indicates an unhealthy culture
and/or chlorophyll a degradation with sample processing
what are the first steps in the chlorophyll a experiment (filtration steps)
add10 mL of the algae culture to filter apparatus, using the hand vacuum pump to separate the algae from the medium
transfer the filter paper with the algae to a homogenizer tube and add 5 mL of absolute methanol
grind the filter paper and methanol using the drill assembly at medium speed for 1 minute
add a new filter paper to the apparatus and put a test tube into the filtration flask to catch the filtrate
pour the homogenized mixture into the filtration apparatus
rinse the homogenizing tube with 3 mL absolute methanol and add to the filtration apparatus
collect the filtrate to use for spectrophotometry
steps of the chlorophyll a experiment involving recording data (spectrophotometry)
add 3 mL of sample to a cuvette
take readings at 650, 665, and 750 nm (use methanal blank at each wavelength)
add 100 microliters of 0.1 N HCl to cuvette and blank
mix and set aside for 4 minutes
add 100 microliters of 0.1 N NaOH to cuvette and blank, then mix
take readings at 665 nm and 750 nm (use blank at each wavelength)
why is HCl added in the chlorophyll a experiment
the acidification converts all chlorophyll a to to phaeophytin
why add NaOH in the chlorophyll a experiment
The NaOH step neutralizes the acidity prior to reading because acidified
samples in methanol affect chlorophyll’s maximum absorption peak
why were readings in the chlorophyll a experiment taken at 750 nm
corrects for turbidity and other pigments that might interfere with the assessment
must subtract the readings at 750 from the readings at 650 and 665 (650-750 and 665-750)
how to determine chlorophyll a to phaeophytin ratio
665 reading before acidification / 665 reading after acidification
(correction for turbidity with 750 reading must be done first)
what is bioassay
an assessment that determines the effect of pollutants on standard test organisms in a lab setting
why were daphnia used in our experiment
are easy to maintain in the lab and do not require animal care permitting
define LC50
50% lethal concentration – this is the
concentration which kills 50% of the organisms over a specified period of time
define no-observable-effect level
the concentration where there was no observable effect on the organisms
normal swimming behavior vs abnormal swimming behavior in daphnia
normal: hop and sinking motion
anormal: slow movement, jittery/fast movement, immobility
what was the purpose of the daphnia bioassay
determine acute and chronic effects of salt (ice melting salt) on daphnia behavior and mortality
what is a standard curve
a graph that is prepared by plotting results for samples of
known concentration (or standards) vs. absorbance
what r2 value range is considered accurate
over 0.95
why use a standard curve
to determine the unknown concentration of samples using the standards
what concentrations are inaccurate in a standard curve
very high or very low concentrations
why are very low concentrations inaccurate
the error of measurement may be too great and result in inaccurate measurements