Module 21 - Alpha Carbon Chemistry: Enols and Enolates
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
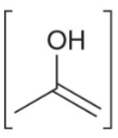
Enol
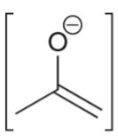
Enolate
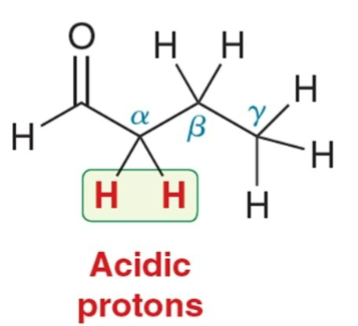
α protons…
the protons attached to an α-carbon
In presence of acid or base, a ketone or aldehyde…
exist in equilibrium with an enol.
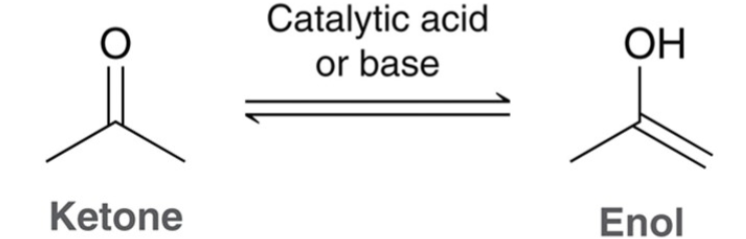
The ketone and enol are…
tautomers of one another
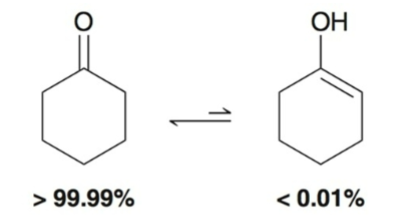
Equilibrium typically favors (ketone or enol)?
ketone
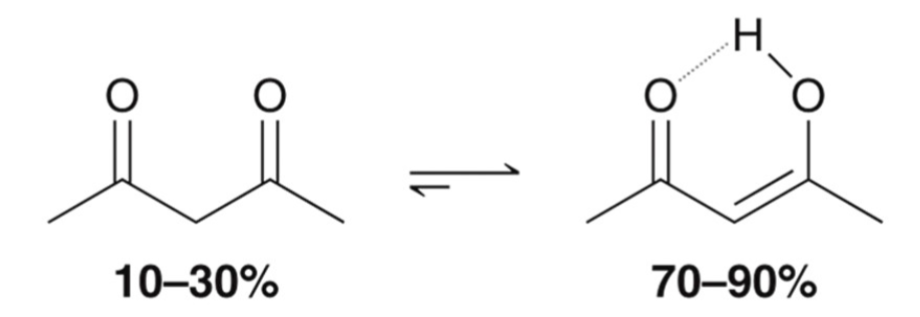
Equilibrium Exceptions: 1,3-diketone enol
enol of a 1,3-diketone is stabilized by conjugation and intramolecular H-bonding; more stable than a typically enol and favored at equilibrium.
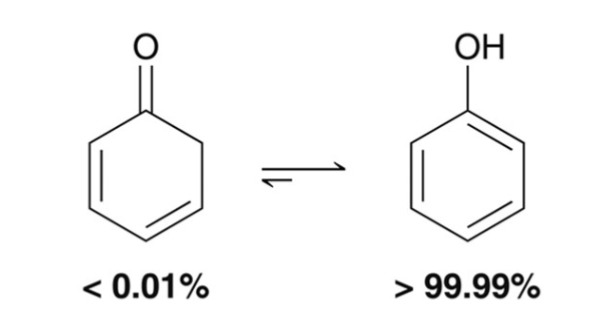
Equilibrium Exceptions: Phenol
enol is vastly favored over the keto equilibrium; phenol is an enol with aromatic stability
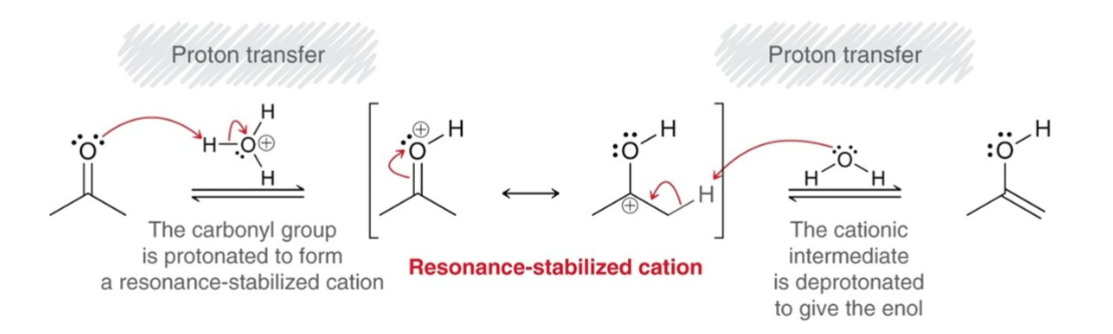
Acidic Tautomerization
Proton Transfer: The carbonyl group is protonated to form resonance-stabilized cation.
Proton Transfer: The cationic intermediate is deprotonated to give an enol.
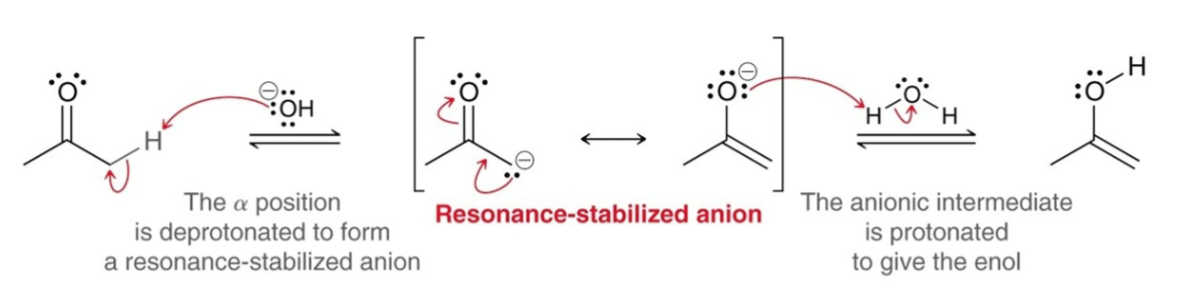
Basic Tautomerization:
Nucleophilic Attack: The α position is deprotonated to form a resonance-stabilized anion.
Proton Transfer: The anionic intermediate is protonated to give an enol.
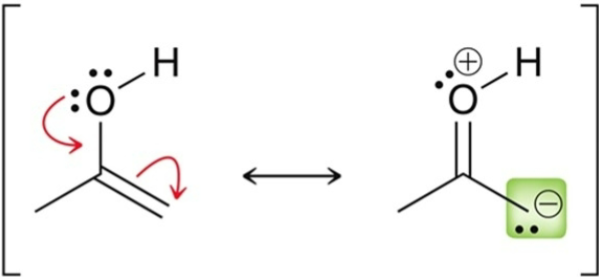
The α position in an enol is…
nucleophilic
In the presence of strong base…
an enolate forms
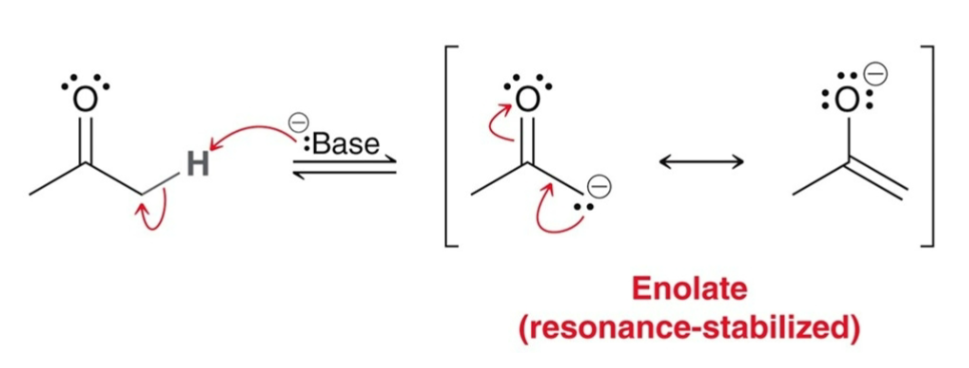
Which is more nucleophilic (enolate or enol)?
the enolate is much more nucleophilic than the enol, as it carries the negative charge.
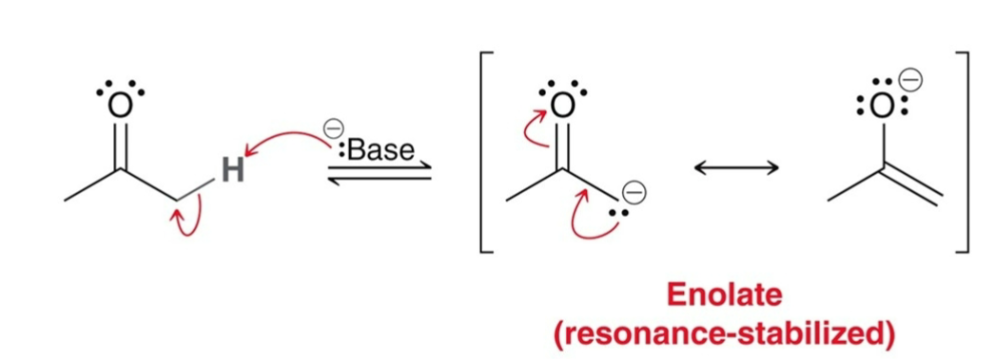
Enolates can undergo…
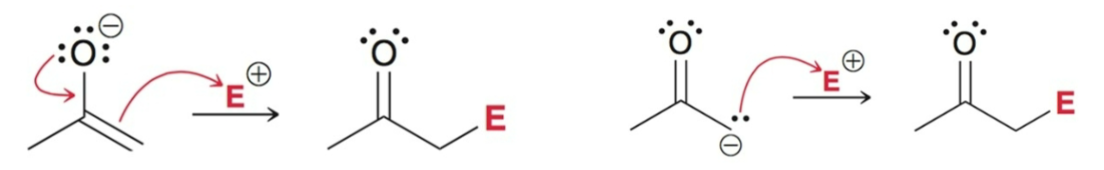
C-attack OR O-attack
Enolates generally undergo…
C-attack (drawn 1 of 2 ways)
Alpha protons are the only acidic protons on an aldehyde or ketone that can…
be removed to form an enolate.
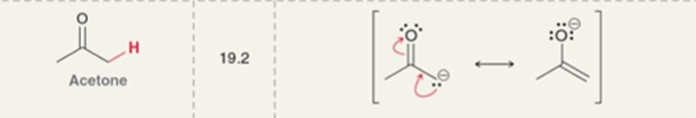
Acetone pKa
pKa = 19.2
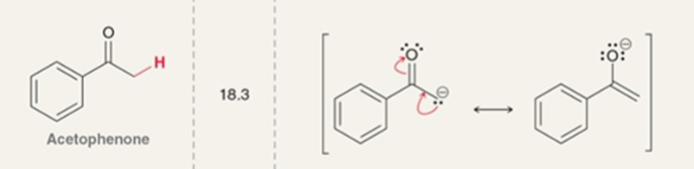
Acetophenone pKa
pKa = 18.3
Acetaldehyde
pKa = 16.7
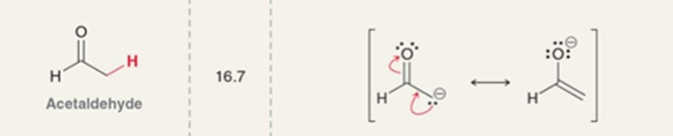
When pKa values of the base and the enolate are similar…
both products and reactants are present in significant amounts
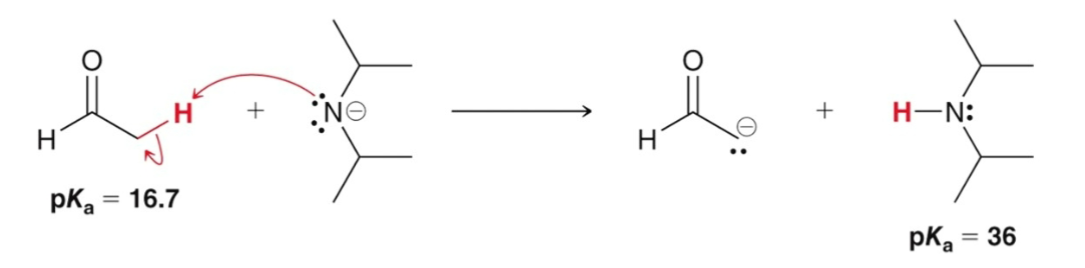
To irreversibly and completely form an enolate…
a stronger base must be used (like NaH or LDA)
LDA
has two isopropyl groups making it a strong base but a bad nucleophile.
When LDA is used to form an enolate…
the amount of ketone present at equilibrium is negligible.
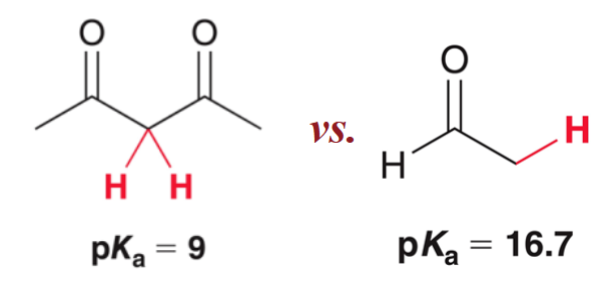
When protons are alpha to two carbonyl groups:
they are much more acidic; increased acidity due to increased stability of the resulting enolate.
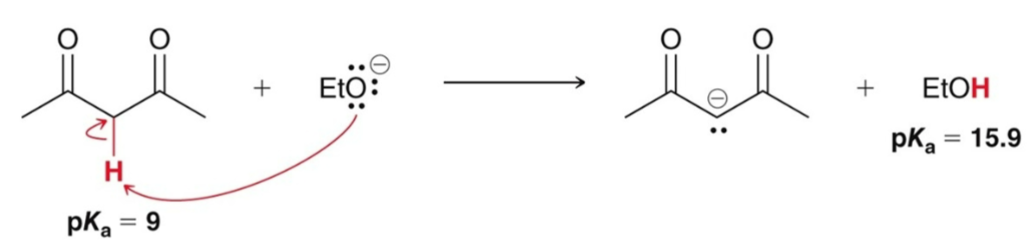
Exception: 1,3-dicarbonyls are acidic enough to be irreversibly deprotonated by…
an alkoxide; no need for NaH or LDA.
Alpha Halogenation - Acidic Conditions
Under acidic conditions, ketones/aldehydes undergo alpha halogenation.
Works with Cl2, Br2, and I2.
Acidic conditions means an enol is the reactive intermediate.
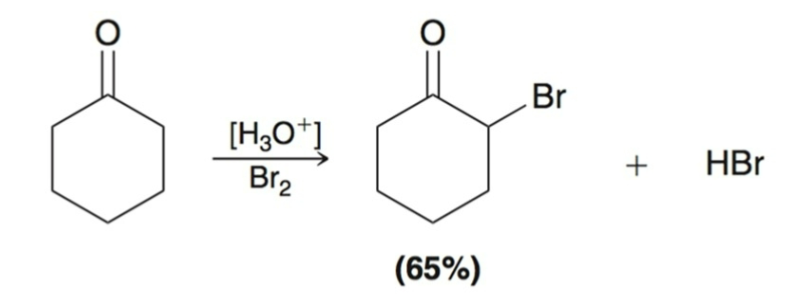
Alpha Halogenation Mechanism Part 1:
Enol Formation
Proton Transfer: carbonyl group is protonated to form a resonance-stabilized cation.
Proton Transfer: cationic intermediate is deprotonated to give an enol.
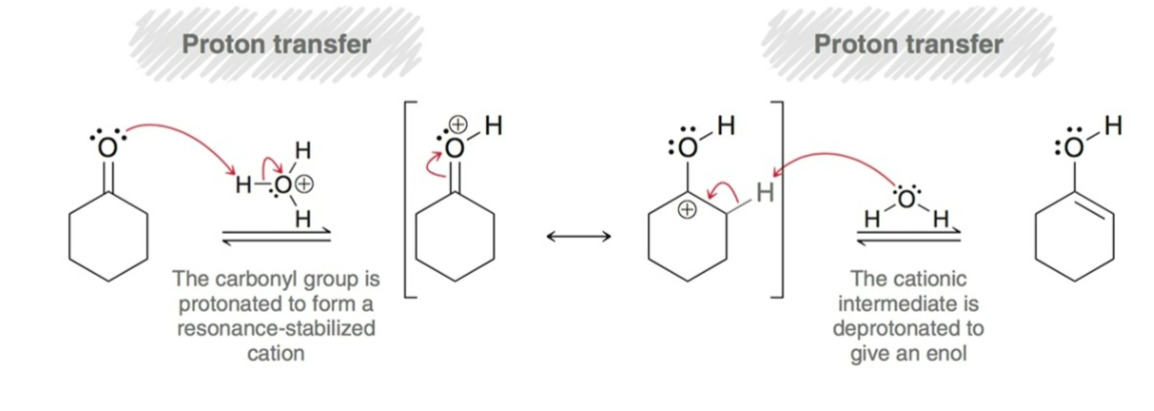
Alpha Halogenation Mechanism Part 2:
Halogenation:
Nucleophilic Attack: enol functions as a nucleophile and attacks molecular halogen.
Proton Transfer: a proton is removed to afford the product.
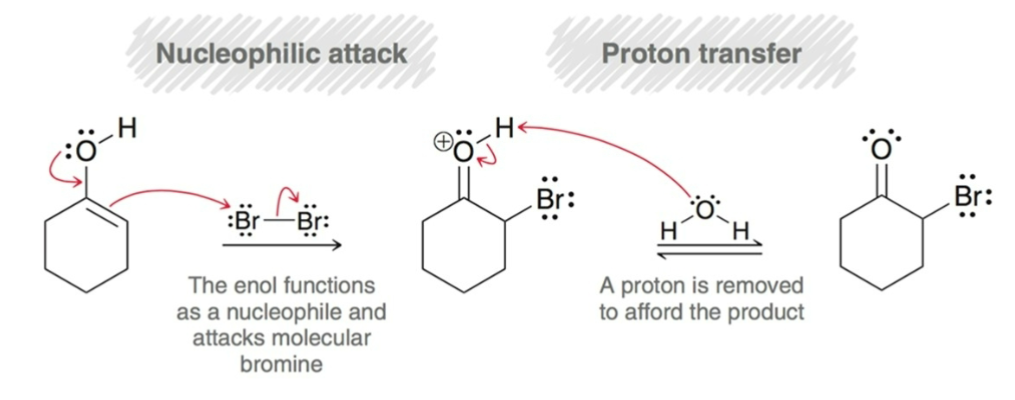
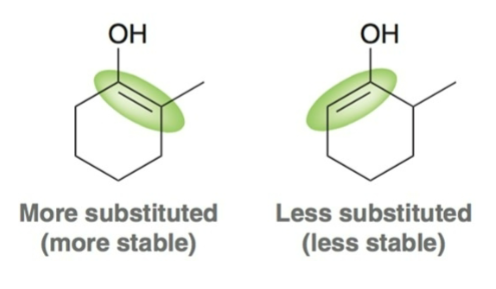
When an unsymmetrical ketones is used, halogenation occurs…
faster at the more substituted carbon.
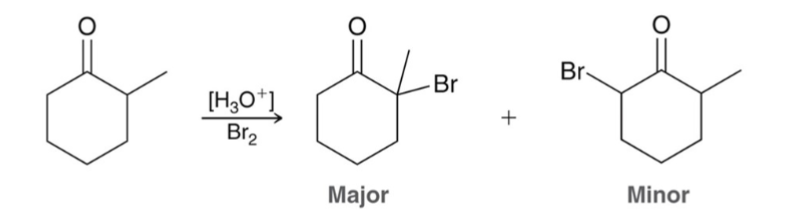
For acidic alpha halogenation, the major product results from the…
more stable/more substituted enol
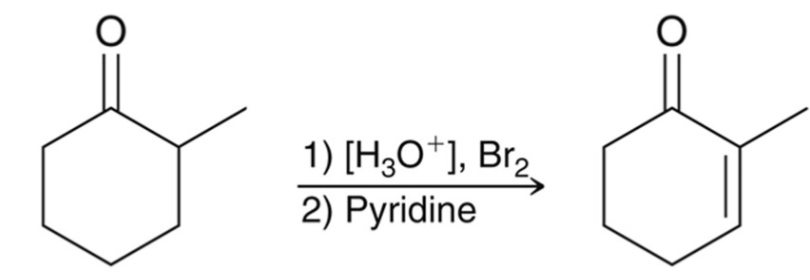
Two Step-Synthesis: Halogenation
Alpha halogenation provides a two-step synthesis for the synthesis of an α, β-unsaturated ketone.
Other bases can be used for the elimination, t-BuOK or Li2CO3.
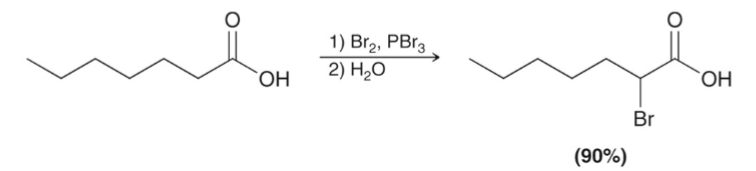
Hell-Volhard-Zelinski (HVZ)
brominates the alpha carbon of a carboxylic acid.
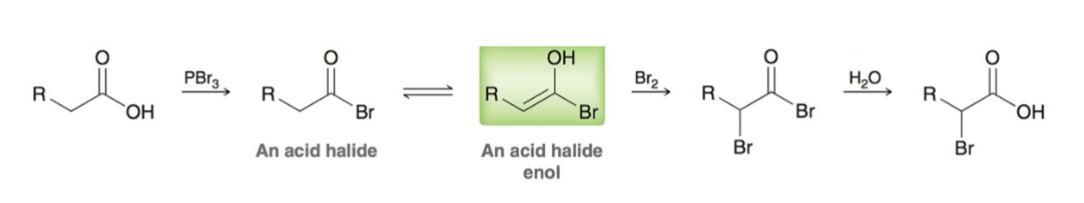
Believed Hell-Volhard-Zelinski (HVZ) Mechanism:
Alpha Halogenation: Basic Conditions
Under basic conditions, an enolates is the reactive intermediate .
Mechanism:
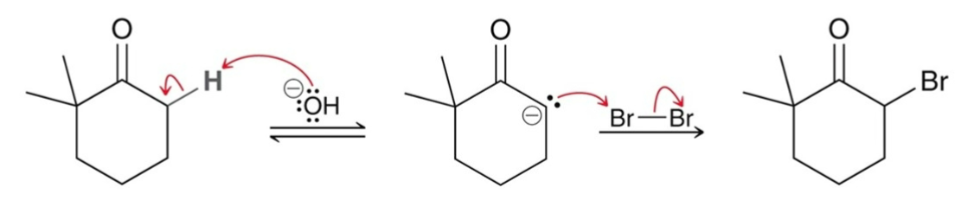
Alpha Halogenation: Under basic conditions, what typically occurs?
Poly-halogenation
once the ketone is brominated, it forms an enolate and brominates again at an even faster rate.
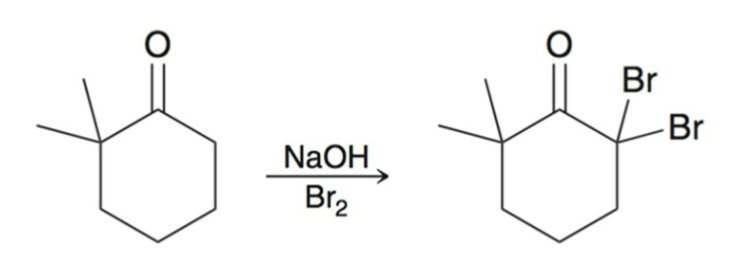
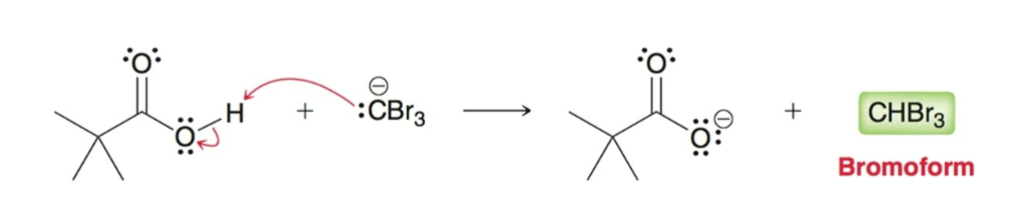
Haloform Reaction
Under basic conditions, methyl ketones are converted into carboxylic acids using excess halogen and hydroxide.
Br2, Cl2, and I2 can be used.
Works best when the other alpha carbon has no alpha protons.
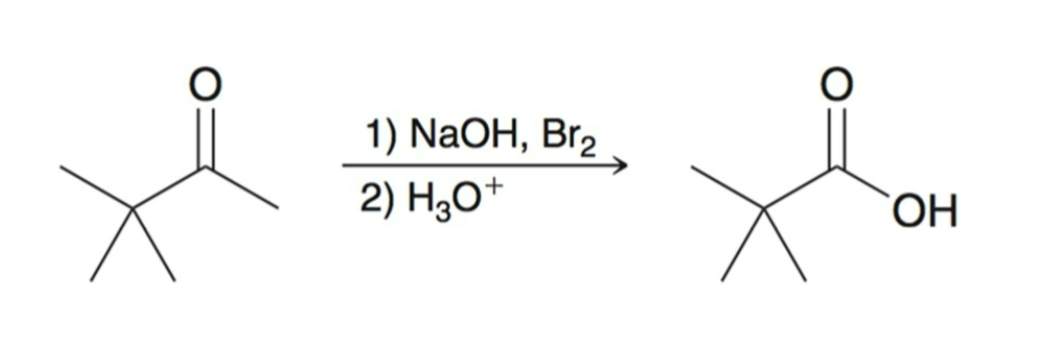
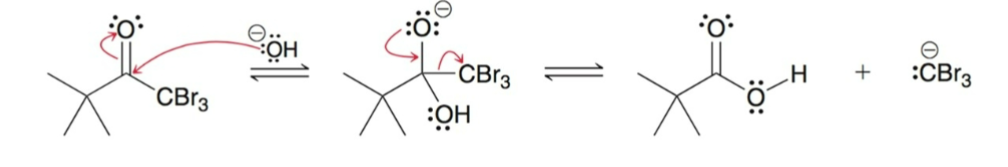
Acyl Substitution
After the 3 alpha protons are replaced, the —CBr3 acts as a good leaving group and acyl leaving group.
The resulting carboxylic acid is deprotonated under basic conditions, this forces the reaction to completion.
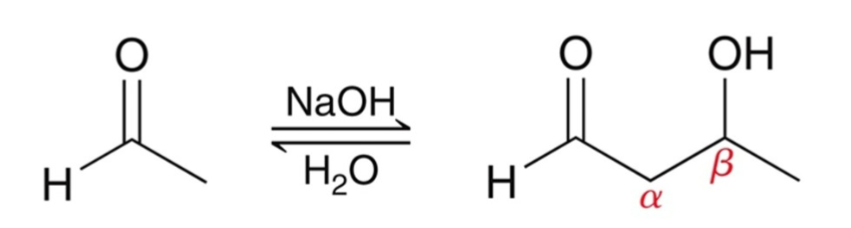
Aldol Addition:
Recall that when an aldehyde is treated with hydroxide (or alkoxide), an equilibrium forms where significant amounts of both enolate and aldehyde are present.
If enolate attacks the aldehyde, an aldol addition occurs.
Product features both aldehyde and alcohol groups.
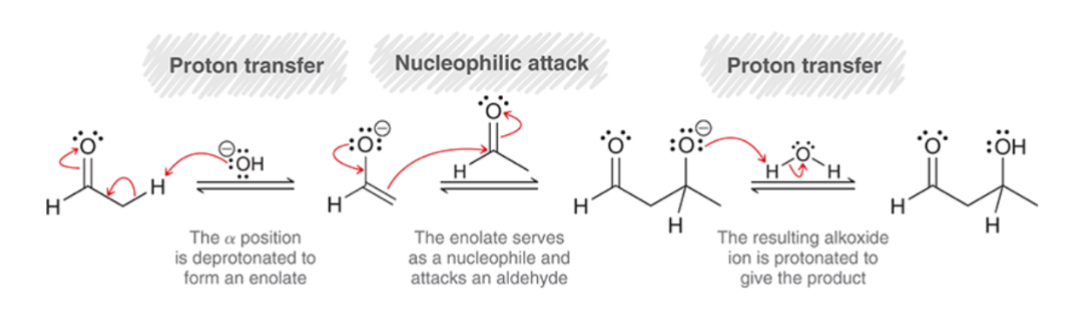
Aldol Addition Basic Conditions Mechanism:
Under basic conditions, enolate is the reactive intermediate.
Proton Transfer: the α position is deprotonated to form an enolate.
Nucleophilic Attack: enolate serves as a nucleophile and attacks the aldehyde.
Proton Transfer: the resulting alkoxide ion is protonated to give the product.
Aldol Addition: Equilibrium
for most simple aldehydes, aldol product is favored.
for most ketones, the aldol product is not favored.
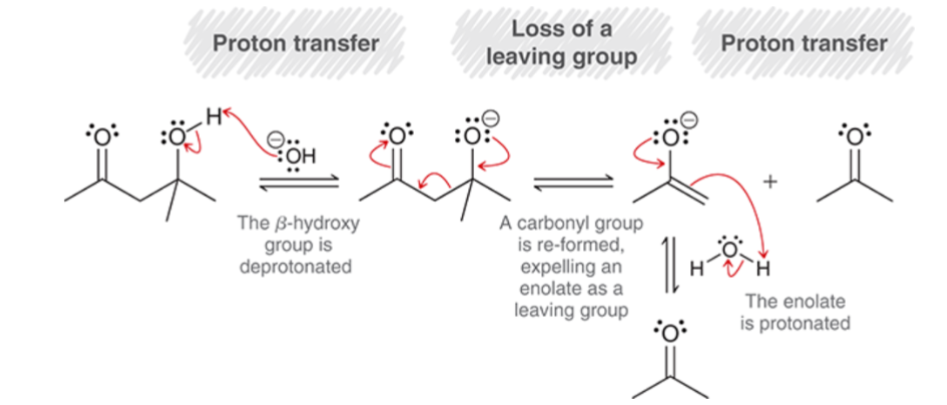
Retro-Aldol Reaction Mechanism:
reverse of an aldol addition
Proton Transfer: the β hydroxy group is deprotonated.
Loss of a Leaving Group: a carbonyl group is reformed, expelling an enolate as a leaving group.
Proton Transfer: the enolate is protonated.
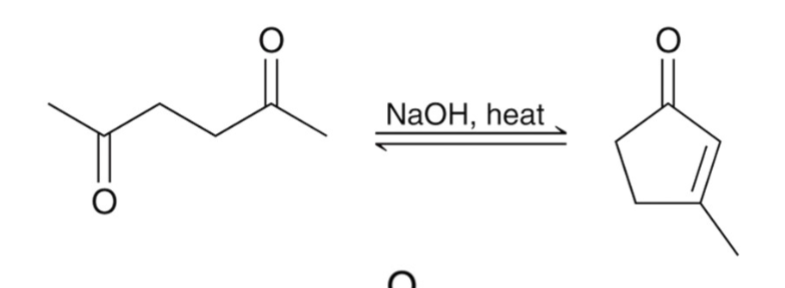
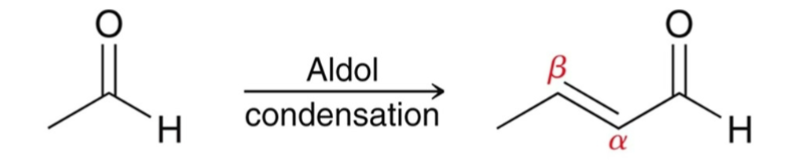
Aldol Condensation
when an aldol product is heated under acidic or basic conditions, an α, β-unsaturated carbonyl forms.
Aldol condensation occurs when an aldol addition is performed at elevated temperatures.
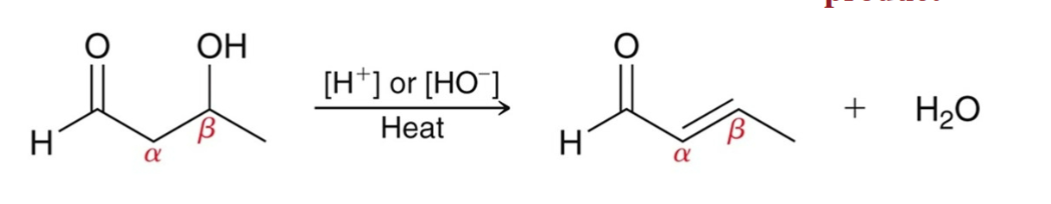
Aldol Condensation Mechanism: Part 1
Aldol Addition
Proton Transfer: The α position is deprotonated to form an enolate.
Nucleophilic Attack: The enolate serves as a nucleophile and attacks the aldehyde.
Proton Transfer: The resulting alkoxide ion is protonated.
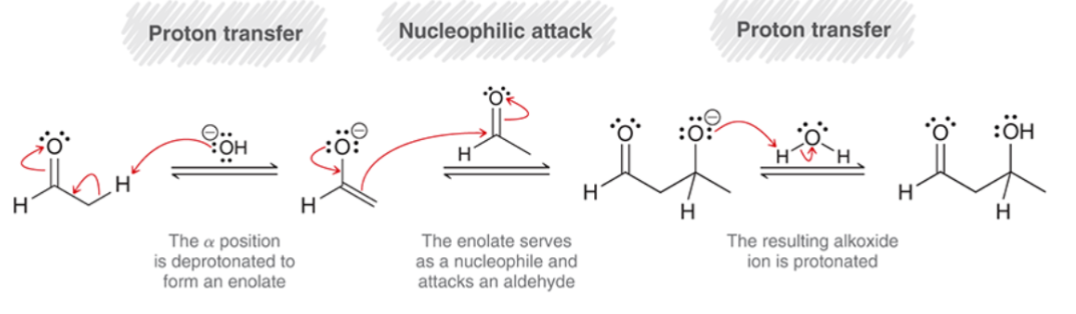
Aldol Condensation Mechanism: Part 2
Elimination of H2O
Proton Transfer: The α position is deprotonated to form an enolate.
Loss of Leaving Group: Hydroxide is ejected to afford the product.
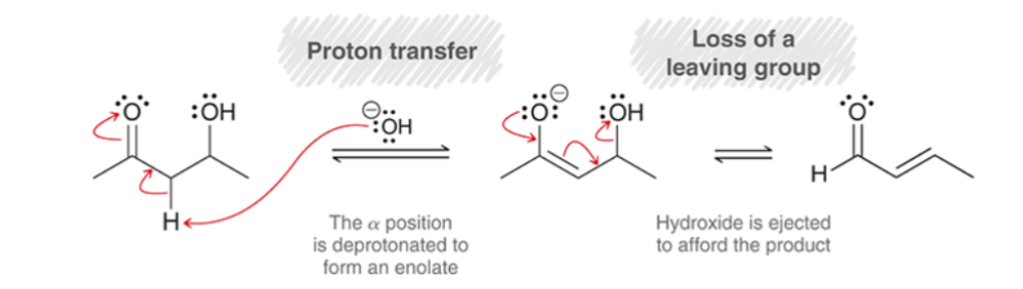
Aldol Addition/Condensation Sterics:
when two stereoisomeric pi bonds can be formed, the product with fewer steric interactions is the major product.
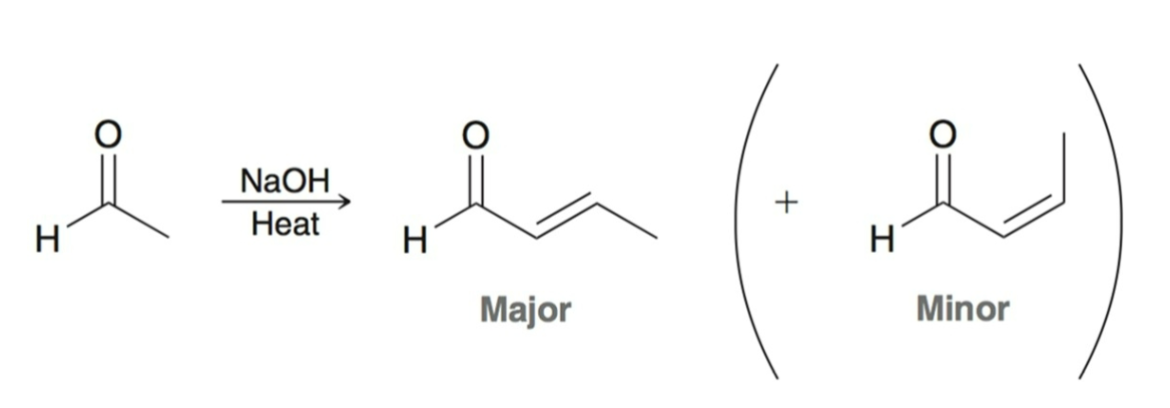
Aldol Addition/Condensation: Isolating Product
B/c aldol condensation is favored, often it is impossible to isolate the aldol addition product.
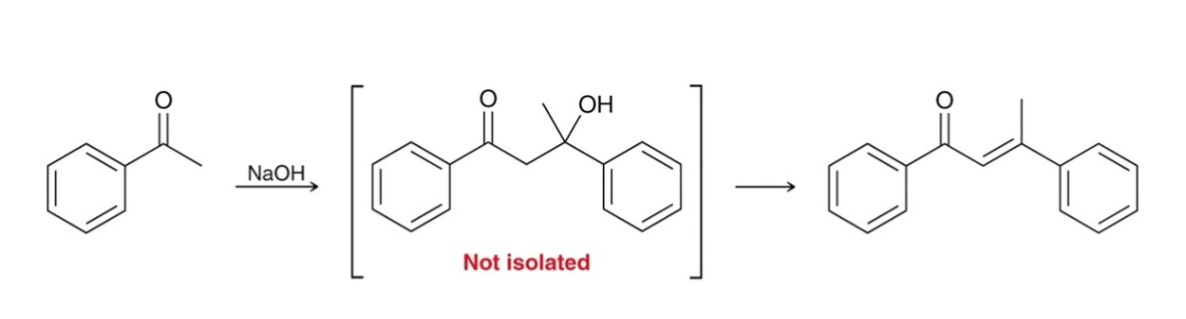
Aldol Addition/Condensation: Yields
yields for condensation are typically much greater than yields for the aldol addition
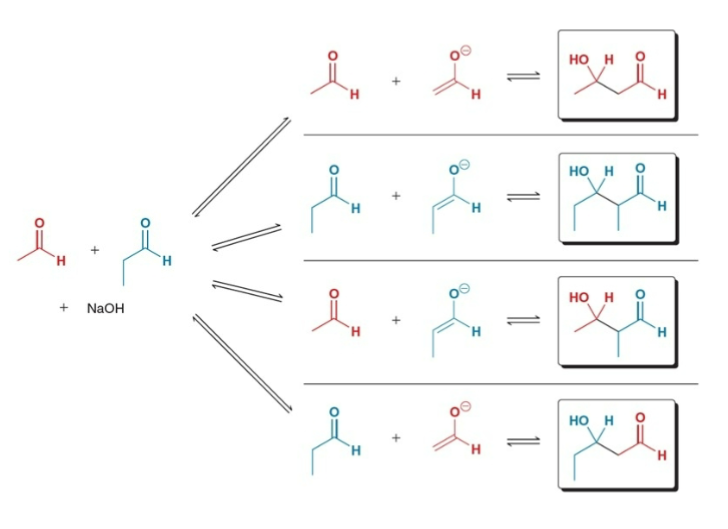
Crossed Aldol Reaction:
Two different aldehydes/ketones react in a crossed aldol reaction (or a mixed aldol reaction).
ex. 2 different aldehydes yield 4 possible aldol products
Crossed aldol reactions are only practical if the number of products can be minimized, which is achieved in one of two ways:
One of the substrates is relatively unhindered and without alpha protons.
-possible when only one compound can form an enolate and the other compound reacts with enolate faster.
Use LDA as a base.
-one compound is completely converted to enolate with LDA.
-the other carbonyl is slowly added to the enolate.
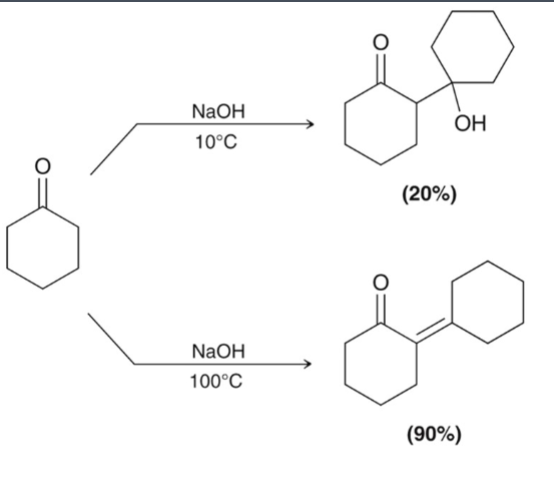
Intramolecular aldol reactions form…
cyclic compounds
one group forms an enolate that attacks the other group.
only 5- and 6-membered rings form well this way.
Claisen Condensation:
Esters undergo reversible condensation reaction.
Simply a nucleophilic acyl substitution reaction
nucleophile = enolate of an ester, electrophile = ester
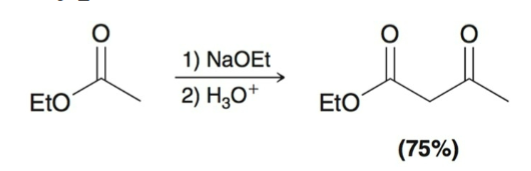
Claisen Condensation Mechanism:
Proton Transfer: the α position is deprotonated to form an ester enolate.
Nucleophilic Attack: the enolate serves as a nucleophile and attacks an ester, forming a tetrahedral intermediate.
Loss of Leaving Group: the carbonyl group is re-formed by ejecting an alkoxide ion.
Proton Transfer: the α position is deprotonated to form a highly stabilized enolate.
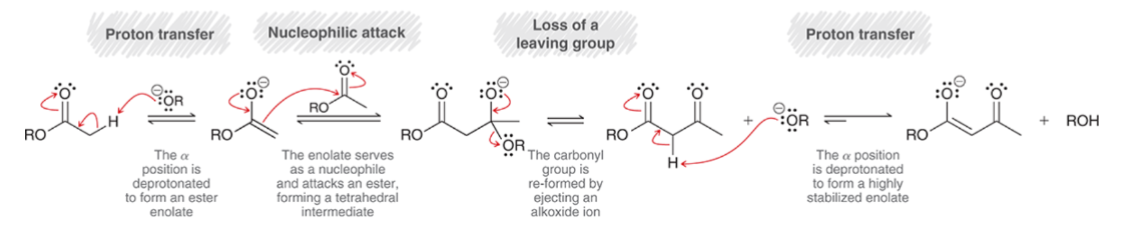
Claisen Condensation: Acidic workup is necessary to…
obtain the neutral product
Use ammonium chloride (NH4Cl) in water for acidic workup to avoid ester hydrolysis.
Claisen Condensation: Limitations
Starting ester must have two alpha protons, because removal of the second proton by the alkoxide ion is what drives equilibrium forward.
Hydroxide cannot be used as the base to promote Claisen Condensations; otherwise ester hydrolysis occurs.
An alkoxide that matches the -OR group of the ester is needed, otherwise transesterification occurs
Crossed Claisen condensations are only useful if one of the following criteria is met:
one ester has no α protons.
perform directed Claisen condensation with LDA as a base
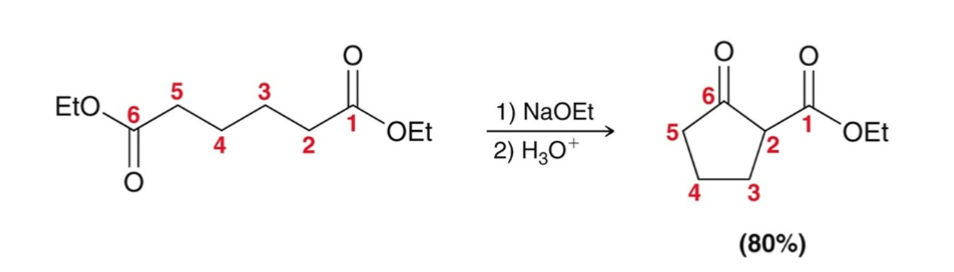
Dieckmann Cyclization:
Intramolecular Claisen condensations
Like with aldol reactions, Dieckmann cyclization prefers the formation of 5- and 6-membered rings.
Alkylation of the Alpha Position
the alpha position can be alkylated when an enolate is treated with an alkyl halide.
the enolate attacks the alkyl halide via a SN2 reaction.
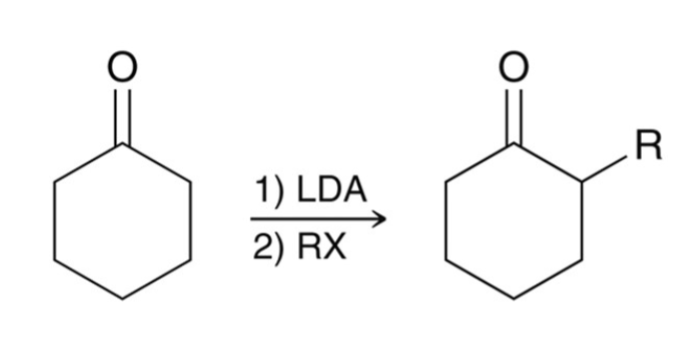
Alkylation of the Alpha Position: Regioselectivity
Two different enolates can form.
Kinetic enolate favored by irreversible conditions; less substituted, less stable, but forms faster; use LDA at low temperatures (sterically hindered base).
Thermodynamic enolate favored by reversible conditions; more substituted/stable enolate; use NaH at room temperature (nonsterically hindered base).
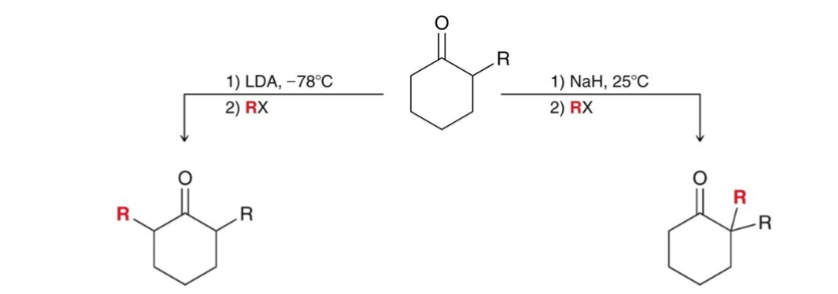
Malonic Ester Synthesis
alkyl halide is converted into a carboxylic acid with two additional carbons.
diethyl malonate is the starting material
Malonic Ester Synthesis Mechanism:
diethyl malonate is reacted with a base to form the corresponding enolate.
the enolate is then reacted with the alkyl halide.
both esters are then hydrolyzed (-OR to -OH)
one of the resulting carboxylic acid groups is then decarboxylated with heat.
-1,3-dicarboxylic acids undergo decarboxylation in a pericyclic process.
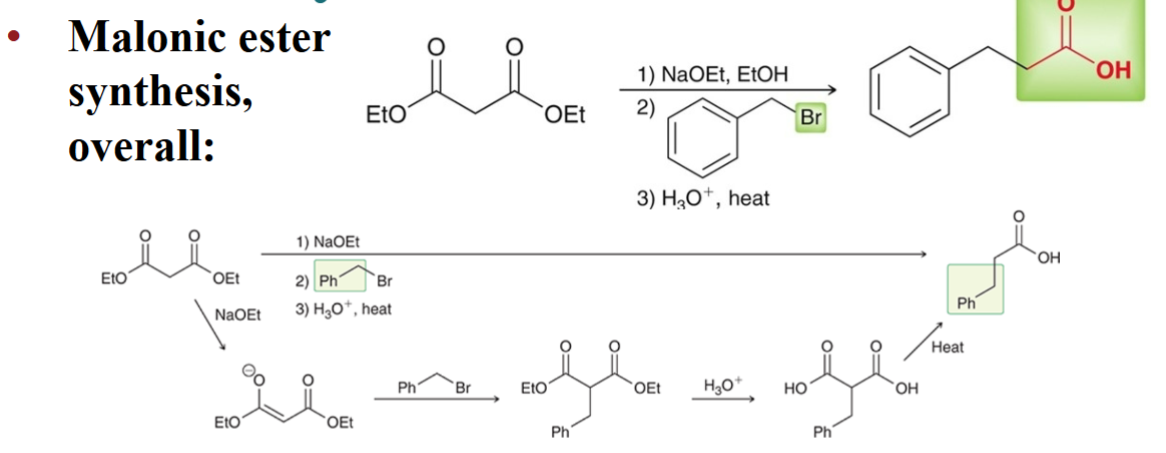
Diethyl malonate can also be…
dialkylated
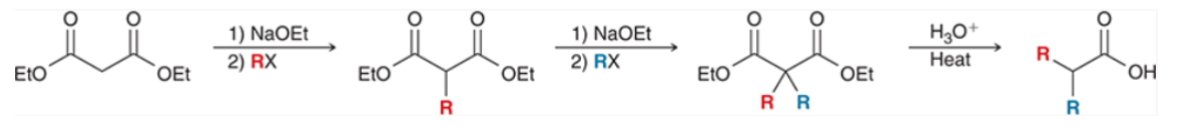
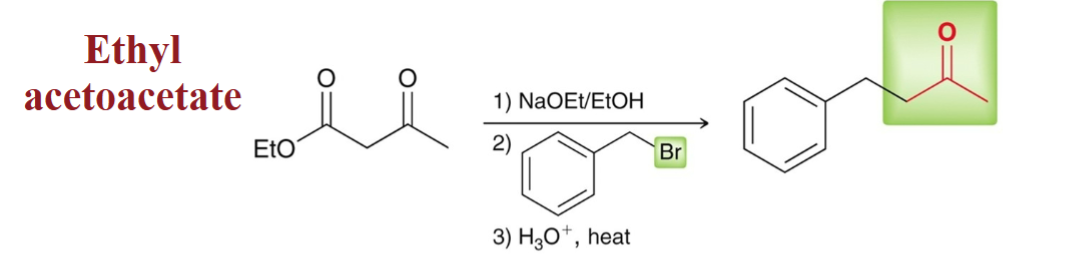
Acetoacetic Ester Synthesis
analogous to the malonate synthesis
converts an alkyl halide to methyl ketone with 3 new carbon atoms
α, β-unsaturated carbonyls are made via…
aldol condensation
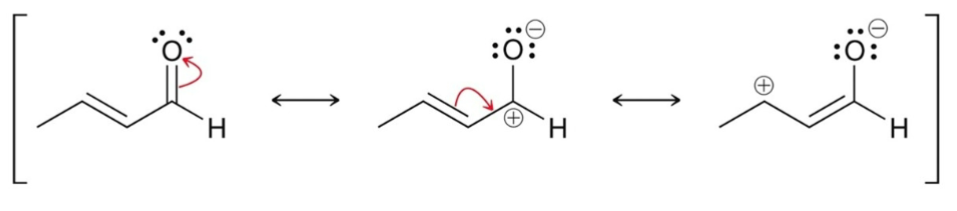
α, β-unsaturated carbonyls have how many resonance contributors?
3
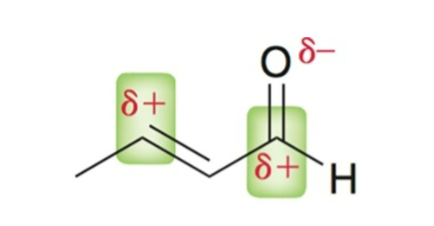
In α, β-unsaturated carbonyls, the β-carbon and carbonyl carbon are…
electrophilic; either carbon can be attacked depending on the nucleophile.
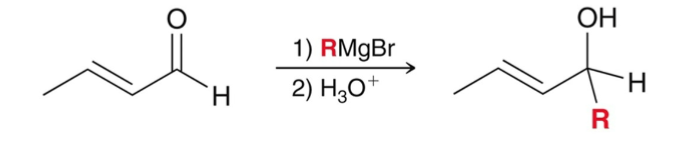
Grignard Reagent (RMgBr)
usually attack the carbonyl position
1,2-addition
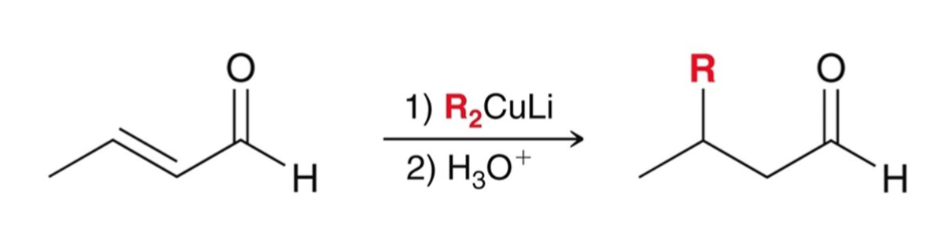
Conjugate Addition: Gilman Reagent (R2CuLi)
usually attacks the β-carbon position
1,4-addition or conjugate addition
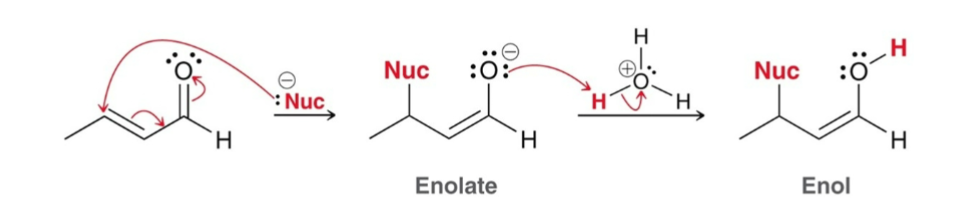
General Conjugate Addition Mechanism:
Nucleophilic Attack: nucleophile attacks the β-carbon position and enolate forms.
Proton Transfer: oxygen is protonated, forming an enol
The enol then tautomerizes to the aldehyde/ketone.
Nucleophiles favor 1,4-addition (thermodynamic product):
H2N-, R2N-, CN-, RO-, Cl-, Br-, I-, RCOO-, HO-, enolate, R2CuLi
Nucleophiles favor 1,2-addition (kinetic product):
LiAlH4, NaBH4, wittig reagent, RMgBr, RLi
Stronger nucleophiles tend towards…
1,2-addition
Less reactive nucleophiles tend to do…
conjugate addition
Typical enolates often give a mixture of…
1,2-additon and 1,4-addition products
Stabilized enolates give…
1,4-addition exclusively
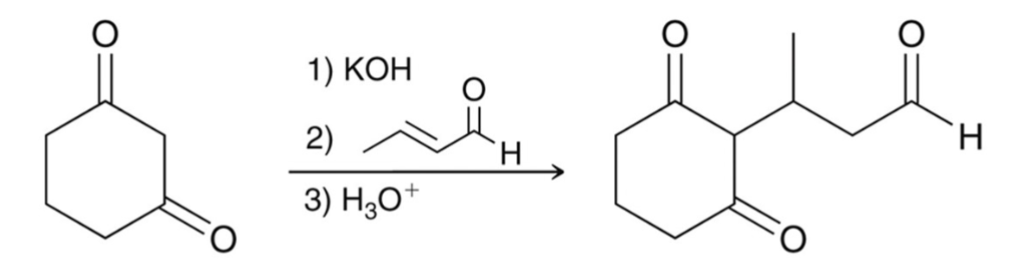
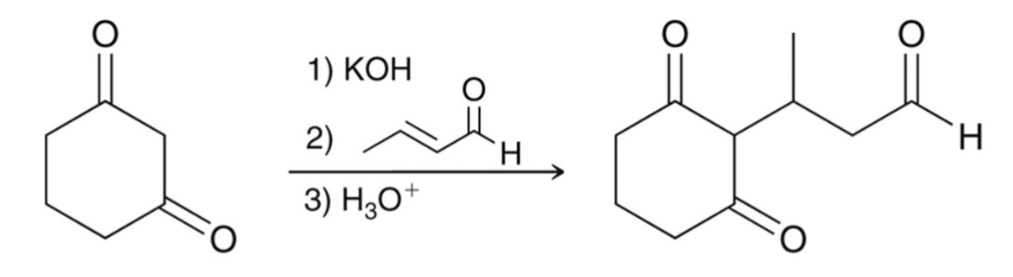
This conjugate (1,4) addition is called a…
Michael Reaction
Michael Donor
nucleophile that does conjugate addition
Michael Acceptor
the α, β-unsaturated carbonyl
Enamines behave like…
enolates (nucleophilic); enamine is less nucleophilic and is Michael donor.
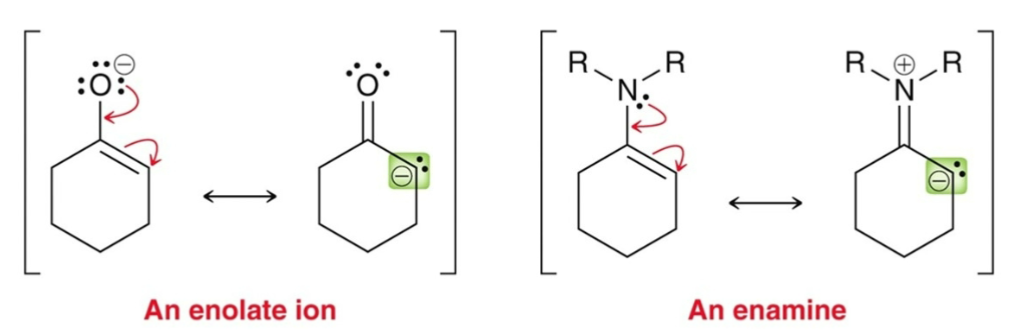
Stork Enamine Synthesis:
3-step process:
Formation of enamine
Michael addition
Hydrolysis
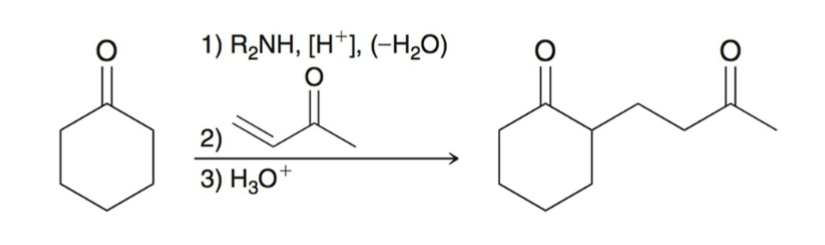
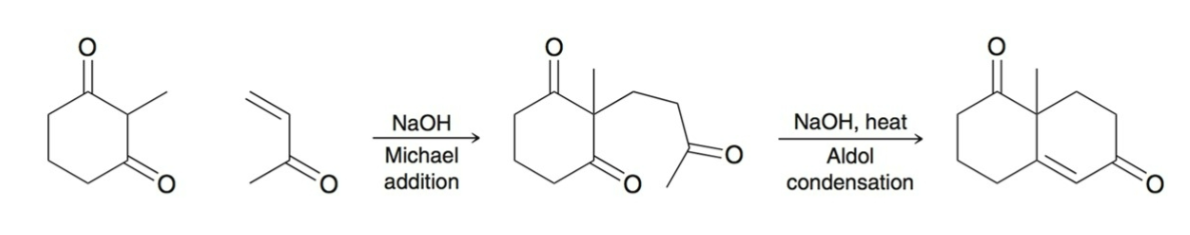
Robinson Annulation:
two step process for forming a ring:
Michael Addition
Intramolecular aldol condensation