Alkanes and Conformations
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
alkanes
molecules with carbons and hydrogens bound together by only single bonds
All carbon atoms in alkanes are sp3 hybridized, meaning each is bound to 4 hydrogen atoms.
Can have alkanes with straight chains, branched chains, and cyclic alkanes
alkane names in ascending order with their substituent names
1 carbon: methane methyl.
2 carbons: ethane ethyl
3 carbons: propane propyl
4 carbons: butane butyl
All you do as you go up is add a CH2 group to the substituents
conformation
different ways to arrange the atoms of a molecule. Usually involves rotating an atom or atoms about a sigma bond.
Recall that sigma bonds allow for free rotation while pi bonds are more rigid and do not allow free rotation because they risk losing wave function overlap.
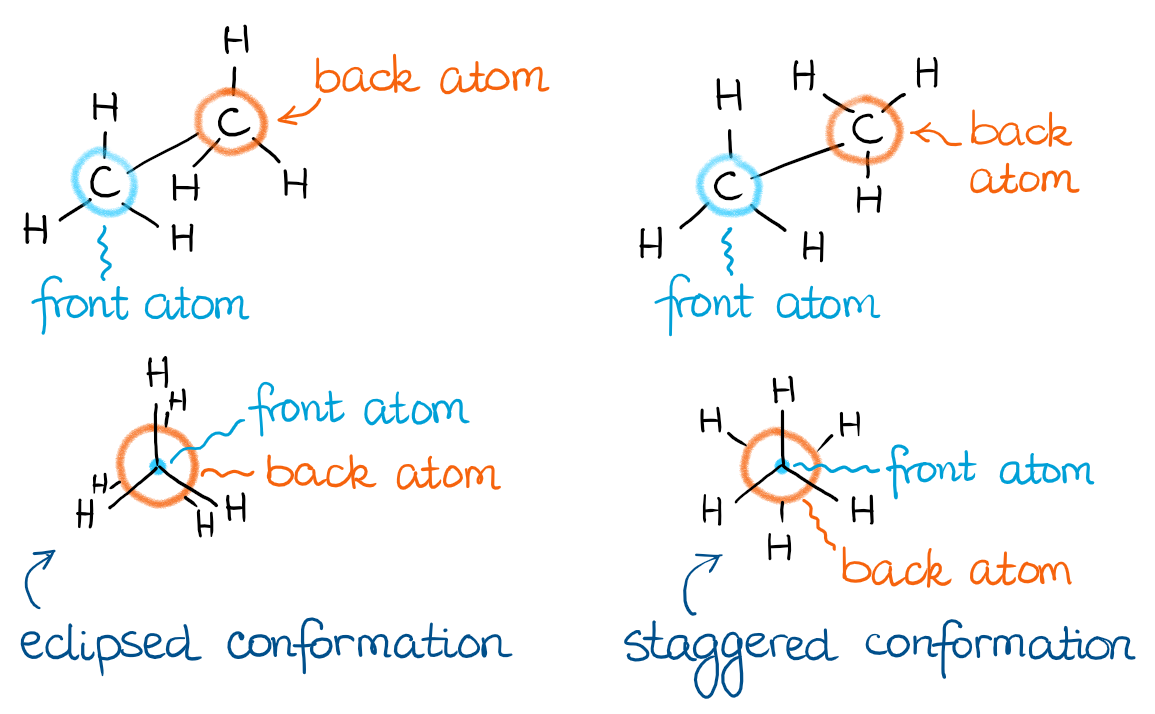
Newman projection
is a different way to look at the various conformations of a molecule.
You act like you are cutting a disk in the middle of the molecule, and look through that disk lengthwise across a bond at the atoms. Kind of like they are in front of you, like through a mirror.
Front (usually carbon) atom is drawn as a dot in the middle of the larger circle. Then the other carbon atom is like behind it, where we cannot see it.
They usually prefer for the part of the molecule on the left of the disk to be on the lines connected to that middle circle.
torsion angle
the angle between an H atom in the front and an H atom in the back. Always in a clockwise direction
the two main conformations about an sp3-sp3 bond
staggered and eclipsed.
Staggered: the torsion angle is 60 degrees. The H atoms of the carbon in the back are halfway between the hydrogens in the front.
Eclipsed: the torsion angle is 0 degrees. The H atoms of the carbon in the back are kind of on top of the H atoms of the front carbon. The H atoms in the front block the view of the H atoms in the back.
torsion energy diagram
an energy diagram with angles on the x axis and energy on the y-axis. Shows the energies of the bond angles, which each correspond to a certain conformation.
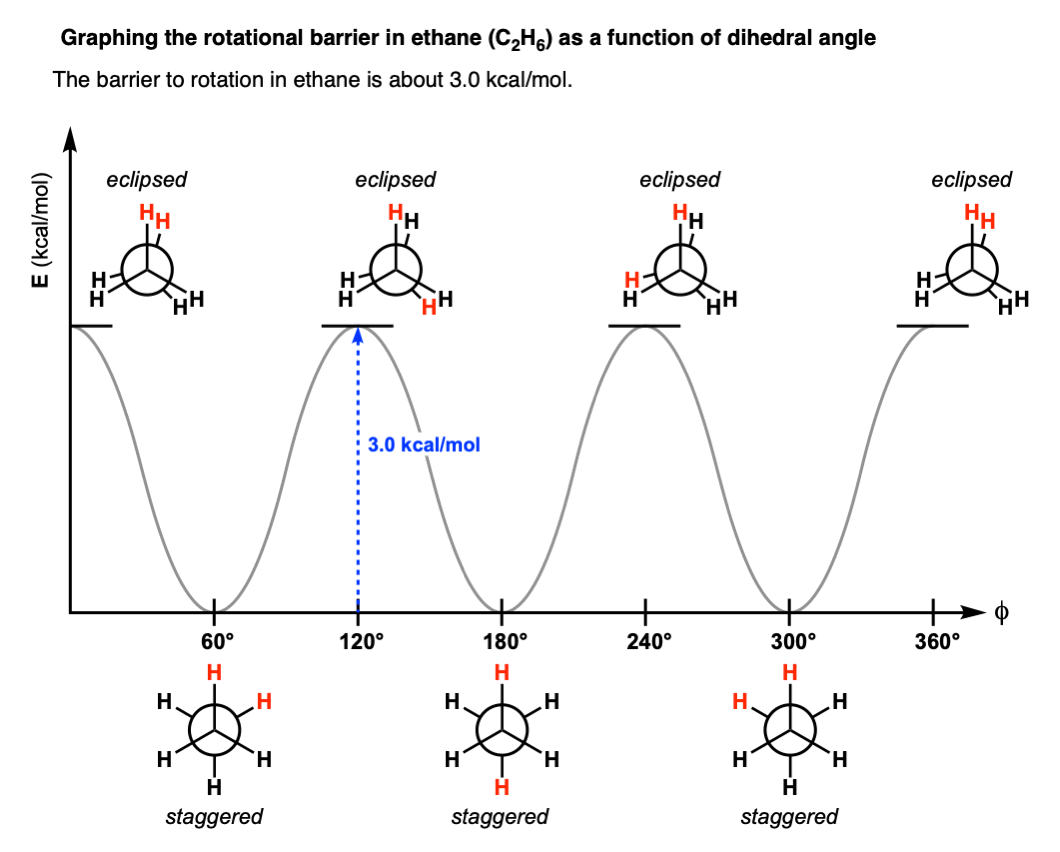
stability of staggered vs eclipsed conformation
the staggered conformation is more stable than the eclipsed conformation. This can be seen on a torsion energy diagram, as all of the angles that correspond to staggered positions are lower in energy than those that correspond to eclipsed positions.
VSEPR explanation for stability of staggered position
VSEPR explains that staggered positions are more stable because they minimize electron pair repulsion. In staggered positions, the Carbon and Hydrogen atoms are more far away from each other than they are in the eclipsed position. So, the eclipsed position has more electron-pair repulsion, and is thus less stable and higher in energy.
FMO explanation for stability of staggered position
FMO theory states that the staggered position is more stable because it promotes molecular orbital overlap and bonding between the filled sigma MO and the empty sigma antibonding MO. This increased overlap makes the molecule more stable and decreases the energy difference between the HOMO and LUMO, which also stabilizes the molecule.
The eclipsed position on the other hand minimizes this MO overlap, thus increasing the energy difference between the HOMO and LUMO and destabilizing the molecule.
energy barriers to rotation
different eclipsed interactions have different energy barriers to rotation/use up more energy.
4 different conformations of butane
once you get to butane (4 carbons), there are 4 possible conformations.
Eclipsed, torsion angle is equal to 0. This conformation has the highest energy barrier because the methyl groups of the front carbon and back carbon are positioned right behind each other and have a lot of repulsion.
Staggered, torsion angle = 60. This is the conformation where the methyl group of the carbon in the back is in between the methyl group of the carbon in the front and the H in the front. This conformation is also called gauche +
Eclipsed, torsion angle - 120. Now the atoms behind each other are CH3 and H, which has less repulsion than the first eclipsed position, but still has some repulsion.
Anti (another staggered conformation) torsion angle = 180. This is the most ideal conformation because the two methyl groups (one in front, one in back) are 180 degrees apart, which minimizes repulsion. The energy barrier is 0 kcal/mol.
torsion energy diagram for butane
Peaks are transition states, are unstable, and are not populated. Molecular confirmations do not exist at these peaks. Rather, these peaks represent the barrier of going from one stable conformation to another.
Eclipsed conformations are at the peaks and have the highest energies.
Gauche + and anti conformations are in the valleys, have the lowest energy barriers, and are the most populated.
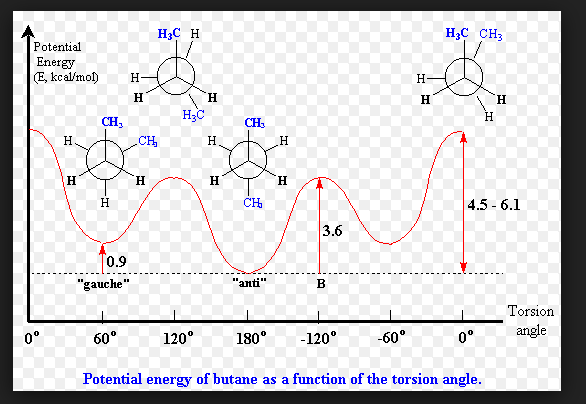
straight chain alkanes usually exist in…
the anti-conformation because it is the most stable. However, all of the conformations are in a dynamic equilibirum, so there can be molecules with one gauche conformation or something like that.
torsion angle strain
applies to eclipse and staggered conformations; strain is caused by electron pair repulsion on atoms that are on top of each other. Eclipsed conformation has a lot of torsion angle strain, while the staggered position minimizes torsion angle strain.
valence bond strain/ring strain
occurs when the angles of the bonds are less than the predicted angles by VSEPR.
In cycloalkanes, because all of the carbons are sp3 hybridized, their bond angles should be 109.5 degrees. However, when the bond angles are less than this, there is valence bond strain.
This strain can also be present when the angle is larger than 109.5 degrees. This is known as large valance bond strain.