Cells and their organelles and stem cells
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Two types of polarity found in cells?
apical and basal polarity
Define apical basal polarity
Apical-basal polarity is the property in epithelial cells where they develop distinct apical (top) and basal (bottom) surfaces, forming an axis that is essential for cell and tissue function
Biomembrane functions
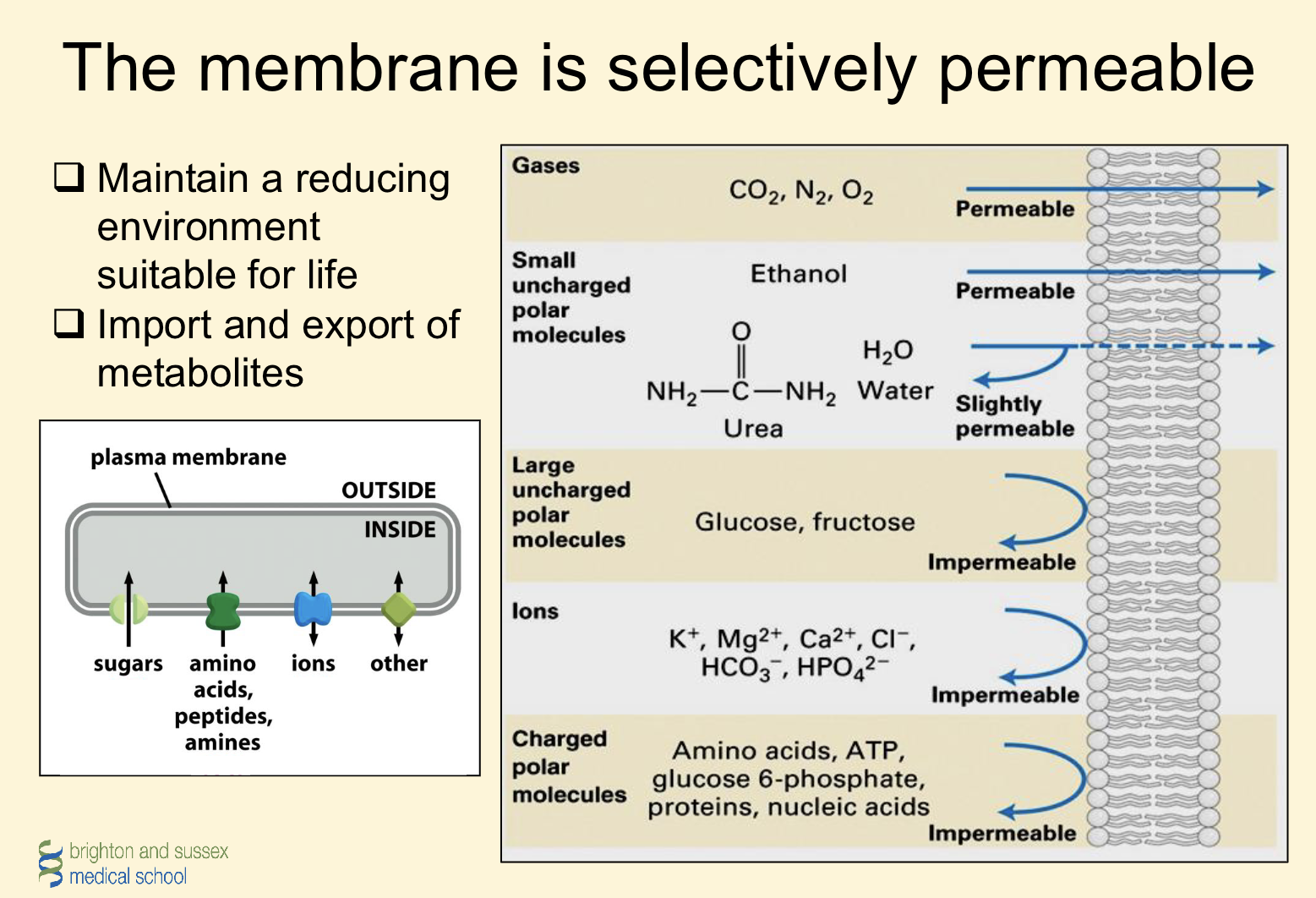
1. Regulate the transport of solutes (import/export)
2. Receive information from microenvironment (receptors)
3. Mediate cell to cell communication
4. Has capacity for expansion/growth (and movement)
Effect of cholesterol affects the fluidity of the
membrane
(more cholesterol reduces fluidity)
4 types of proteins
Transporters, anchors, receptors, enzymes
Example and function: Transporters
Na+ pump
actively pumps Na+ out and K+ into cells
Example and function: Anchors
Integrins - A type of protein found on the surface of cells that helps them attach to, and communicate with, nearby cells
Link intracellular actin filaments to extracellular matrix proteins
Example and function: Receptors
Binds extracellular PDGF to generate intraceulllar signals that cause cells to grow
Membrane is selectively permeable
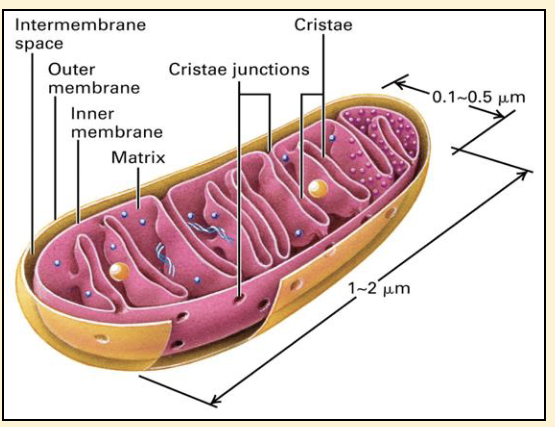
Mitochondria
The ‘energy powerhouse’
❑ site of ATP generation
❑ clustered in cells that have
high energy demands e.g.
muscle cells
❑ Outer membrane is highly
permeable
❑ Inner membrane folded into cristae,
where oxidative phosphorylation
takes place
❑ Matrix a mixture of enzymes
Generation of ATP in the mitochondria
Movement of electrons
coupled to pumping of
protons
❑ Creates a proton motive
force
❑ Electrochemical gradient
generates ATP through
ATP synthase
Endoplasmic Reticulum (ER)
❑ Site of protein synthesis,
protein complex assembly
and modification
❑ Rough ER surrounded by
ribosomes
❑ Smooth ER lipid and
steroid hormone synthesis
❑ Transport of materials
within the cell
❑ Where transported in the cell dependent
on 15-60 aa signal sequences (usually
removed afterwards)
Golgi Apparatus
❑ Modification, packaging and sorting of
proteins and lipids - enhancing complexity
❑ Destined either for secretion or for another
organelle or cell membrane via vesicles
Lysosomes
❑ Vesicles packed with
degradative enzymes
❑ Low pH
❑ Digest and destroy
Peroxisomes
❑ Small vesicles for reactive
H2O2 generation
❑ Oxidation reactions and
breakdown of fatty acids
Main function of cytosol
contains many metabolic pathways, protein synthesis
Main function of nucleus
Contains main genome,DNA and RNA synthesis
Endoplasmic reticulum
Synthesis of most lipids
synthesis of proteins
Main function of golgi apparatus
Modification, sorting, packaging, of proteins and lipids for delivery or secretion
Main function of lysome
Intracellular degradation
Cytoskeleton function
Structural - supports the
plasma membrane
❑ Controls cell shape (and
therefore often function)
❑ Pulls the chromosomes
apart during mitosis
❑ Drives and guides the
intracellular transport of
organelles, proteins
❑ Enables some cells to
move
Three major components of the cytoskeleton
intermediate filament
microtubules
actin filaments
intermediate filaments
❑ 10 nm diameter filaments twisted into ropes to provide tensile strength
❑ Needed to maintain cell shape, flexible, withstand mechanical stress
❑ Made up of a family of fibrous proteins:
- keratin filaments in epithelial cells
- vimentin in many other cells
- neurofilament proteins in neurones
- lamins within the nucleus
Actin filaments
Polymers of actin monomers
❑ Necessary for movement, especially cell surface
❑ Can form contractile bundles and microvilli (strength
in number) – crosslinked bundles and networks
❑ Carry cargo-bearing motor proteins
❑ May associate with myosin to form powerful
contractile structures e.g. Muscle sarcomere
Microtubules
❑ 20 nm diameter polymers of tubulin dimers
❑ Rapidly assemble and disassemble, polar
❑ Organised from structures such as the centrosome
❑ Carry cargo-bearing motor proteins
❑ Important in cell shape and movement (cilia)
❑ Form the spindle in mitosis
Two families of motor proteins
1. Kinesins (out)
2. Dynein (In)
Molecular motors are dependent on
ATP
2 types of motor proteins size and speed
Dynein
0.2- 60 m/sec
Kinesin
(0.02-2 m/sec)
Defining properties of stem cells
❑ They are not terminally differentiated
❑ They can divide without limit
❑ When a stem cell divides, each
daughter can either (1) remain a stem
cell; (2) embark on a course to terminal
differentiation
❑ They are required wherever there is a
recurring need to replace differentiated
cells
❑ They are maintained in stem cell niches
keeping them undifferentiated (wnt
signaling pathway)
Definition of Terminally differentiated
Specialised cells performing a specific
function that cannot proliferate further, will die and will need to be replaced
Definition of stem cell
Relatively undifferentiated self-renewing cell that produces
daughter calls that can either differentiate into more specialized cell types
or can retain the development potential of the parent cell
Definition of pluripotent
Capable of giving rise to any type of cell or tissue (except
the placenta) i.e. an embryonic stem (ES) cell
Definition on multipotent
Capable of giving rise to a discrete range of cells or tissues
i.e. haemopoietic stem cell
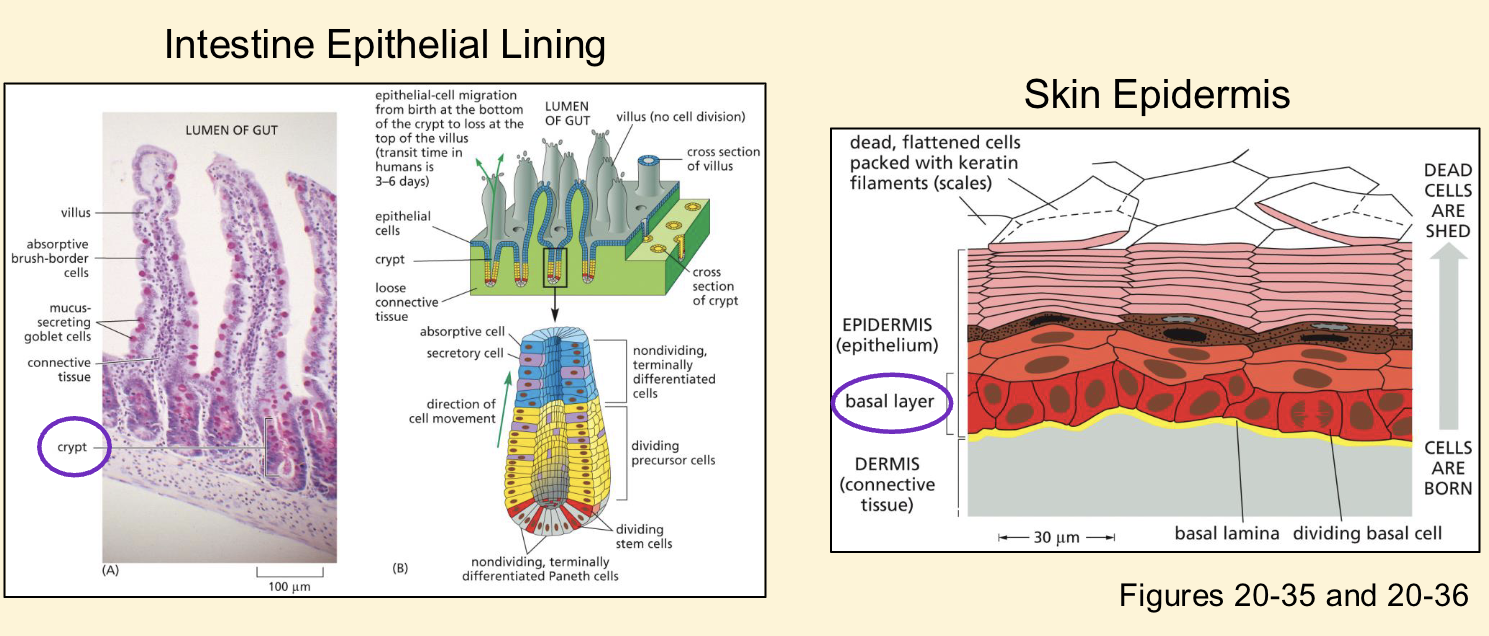
Adult multipotent stem cells features and examples
❑ Required to replace dead terminally differentiated cells
(cell turnover)
❑ Cells of the intestine epithelium replaced every 3-6 days
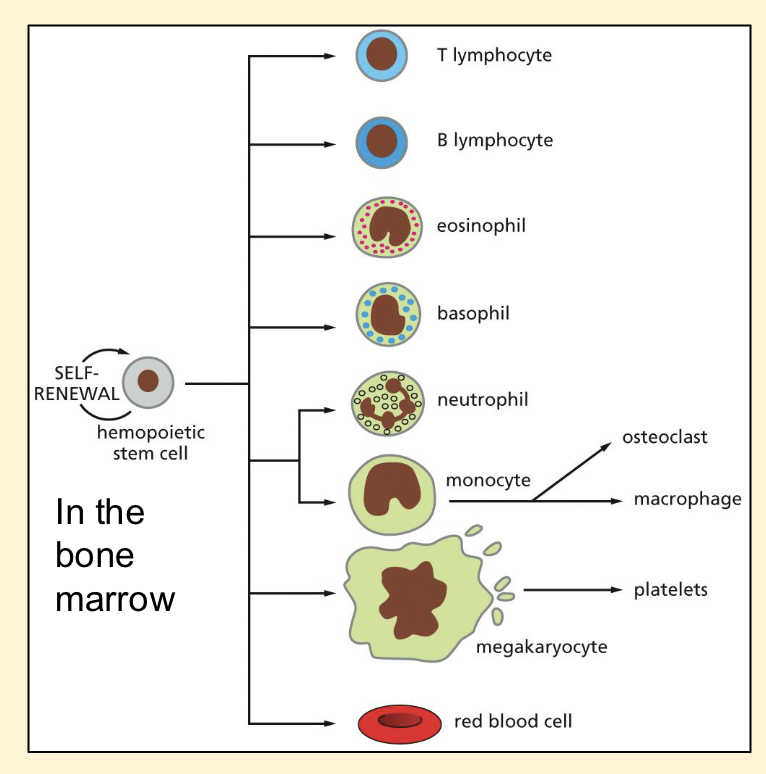
Haemopoietic stem cells features and examples
❑ 2-3 million new erythrocytes are
produced per second in human
adults
❑ Multipotent
stem cells
❑ Huge variety
of cell types
If goes wrong:
❑ anemia (too few red blood cells)
❑ leukopenia (too few white blood
cells)
❑ blood cancers - leukemia,
lymphoma, myeloma - stem cell
transplants possible treatment
option
embryonic stem cells can be used as spare parts
Why and how
Embryonic stem cell (ES) – Undifferentiated cell type derived from the inner cell mass of an early mammalian embryo and capable of differentiating to give rise to any of the specialised cell types in the adult body
❑ Regenerative medicine and
tissue replacement after injury or
disease
❑ From same person, less chance
of tissue rejection
❑ Personalised medicine
❑ Ethical and technological issues,
potential cancer risk
Induced pluripotent stem cells (IPS) summary
Induced pluripotent stem cell (iPS or
iPSCs) – Somatic cell that has been
reprogrammed to resemble and behave
like a pluripotent embryonic stem cell
(ES) through the artificial introduction of
a set of transcriptional regulators
Induced pluripotent stem cells (IPS) advantages
❑ Any cell type could be replaced
❑ Less chance of rejection
❑ Fewer ethical issues
❑ Important for medical research
Induced pluripotent stem cells (IPS) disadvantages
❑ More basic research needed on
developmental pathways
❑ Potential cancer risk
Two ways of cell death
Necrosis, apoptosis
Necrosis (lysis)
❑ Cells simply swell and burst
❑ The membrane is destroyed and cell
contents released into the tissue
❑ Degraded by extracellular enzymes
and phagocytic cells engulf the
remains
❑ Potential damage to neighbouring
cells, trigger inflammatory response
Apoptosis (programmed cell death)
❑ Normal carefully-controlled process
❑ Billions of cells in adult every hour
❑ No damage to neighbouring cells
❑ Internal or external signals activate
Bcl2 family that regulate process
❑ Cell dismantled by caspases, then
phagocytosed