3.1.4.2 - Calorimetry
1/5
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
How do you find the enthalpy of combustion of a liquid?
To find enthalpy of combustion of flammable liquid, burn it in calorimeter
As fuel burns, it heats water → can calculate heat energy absorbed by water using mass of water, temp change and specific heat capacity
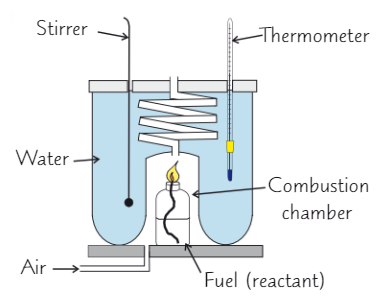
Why are enthalpy of combustion values often inaccurate?
Heat lost to surroundings
Some incomplete combustion
Some fuel lost to evaporation (flammable liquids often volatile)
How do you measure enthalpy changes in solution?
Calorimetry can be used to find enthalpy change for reactions in solutions, such as neutralisation, dissolution (dissolving), displacement
To find enthalpy change of neutralisation reaction, add a known volume of acid to insulated container (e.g. polystyrene cup) and measure temp
Add known volume of alkali and record temp at regular intervals (stir solution to distribute heat)
Find temp change for experiment
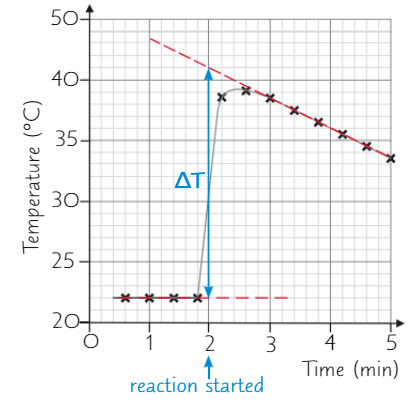
How do you use a graph to find temperature change?
During experiment, record temp at regular intervals, starting 3 mins before reaction begins
Plot graph of results - draw two lines of best fit: one through points before reaction started, and one through points after it started
Extend both lines so they both pass the time the reaction started
Distance between two lines at time when reaction started = accurate temp change
Formula for enthalpy change
q = mcΔT
What does each symbol in enthalpy equation stand for?
q = heat lost/gained (J)
m = mass of water/other solution (g)
c = specific heat capacity of water (4.18 J g-1 K-1)
ΔT = temp change (K)