Intra/Intermolecular forces + Periodic Trends - Chemistry
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Explain the difference between intramolecular and intermolecular forces. Give examples
Intramolecular forces are the forces that hold atoms together within a molecule. These forces are very strong and include ionic bonds, where electrons are transferred between metals and nonmetals; convalescent bonds, where electrons are shared between atoms. Metallic bonds, where electrons are delocalized across metal cations.
Intermolecular forces, on the other hands, exist between separate molecules and are generally much weaker. They play a key role in determining physical properties like boiling points and melting points
Examples include London dispersion forces in non-polar molecules, dipole-dipole interactions in polar molecules, hydrogen bonding in molecules like water and ion-dipole interactions when ions interact with polar molecules
Describe London dispersion forces and when they are strongest.
London dispersion forces are temporary attractive forces that occur when the electrons in two adjacent atoms or molecules happen to be distribution uneven, creating instantaneous dipoles. The forces are present in all molecules but are especially significant in non-polar molecules, which do not have permanent dipoles
The strength of London dispersion forces increases with the umber of electrons in a molecule, the molar mass, and the surface area that can intereact with neighboring molecules.
For example, iodine (I2) has stronger London dispersion forces than fluorine (F2) because it has more electrons and a larger surface area
What is hydrogen bonding, and why does it increase boiling points?”
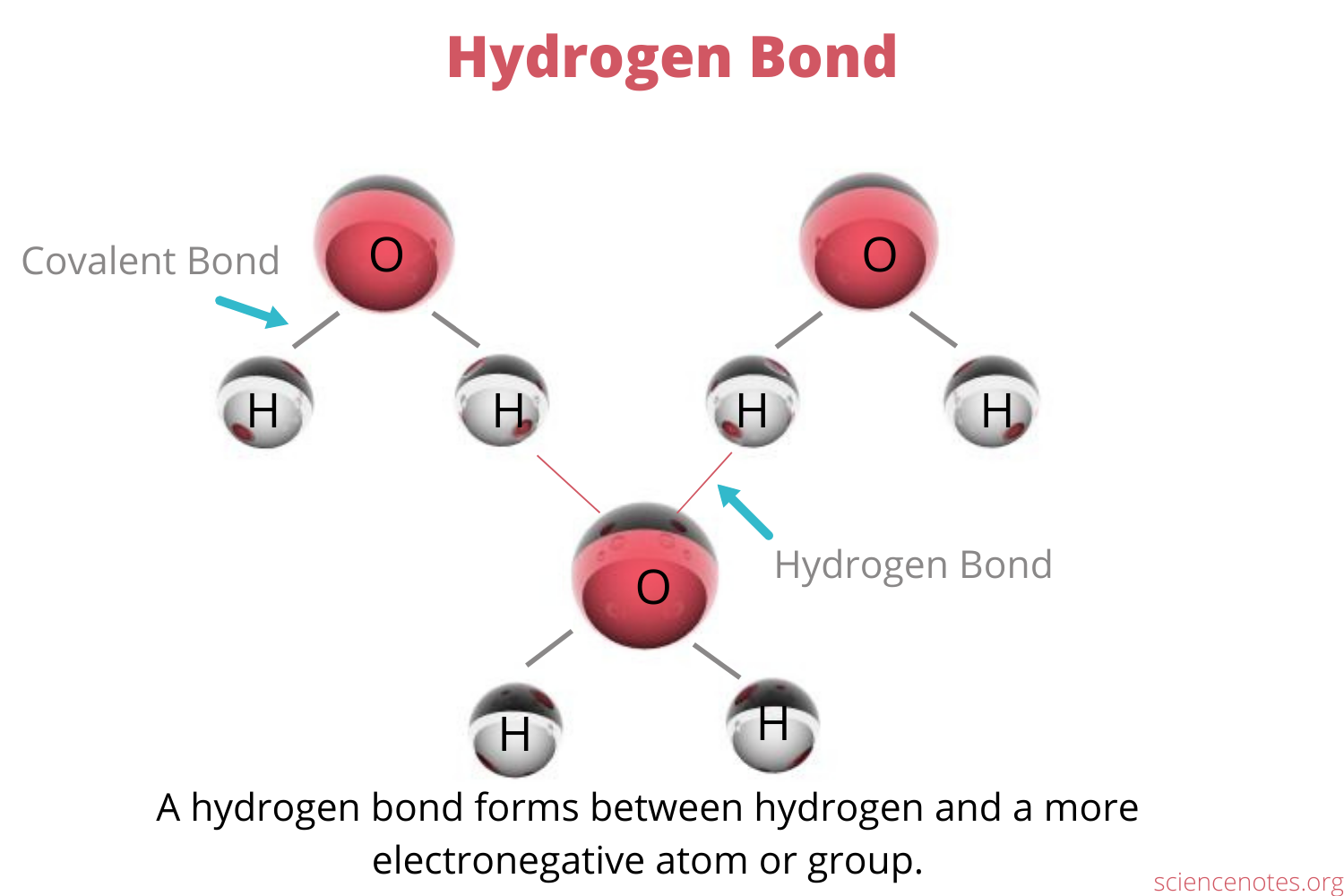
Hydrogen bonding is a special type of intermolecular force that occurs when hydrogen atom is covalently bonded to a highly electronegative atom such as nitrogen, oxygen, or fluorne, and this hydrogen atom is attracted to a lone pairs of electrons on another electronegative atom in a nearby molecule.
Hydrogen bonding is stronger than typical dipole-dipole intereactions and significantly increases the amount of energy required to separate molecules. This is why water, which can form multiple hydrogen bonds, has a much higher boiling point than molecules of similar size that cannot form hydrogen bonds, such as hydrogen sulfide.
Compare CH4, CH3CI, and CH3OH in terms of boiliding point and explain why
The boiling points of CH4m CH3CI, and CH3OH differ primarily due to the types of intermolecular forces present in each molecule. Methane (CH4) is non-polar and experiences only London dispersion forces, which are relatively weak, so it has the lowest boiling point.
Chloromethane (CH3CI) is a polar molecules, so it experiences dipole-dipole interactions in addition to London dispersion forces, resulting in a higher boiling point than methane. Methanol (CH3OH0, however, can form hydrogen bonds because it contains an -OH group, making its intermolecular forces the strongest among the three molecules. Therefore, methanol is the amount of energy required to separate the molecules, which explains the trend in boiling points
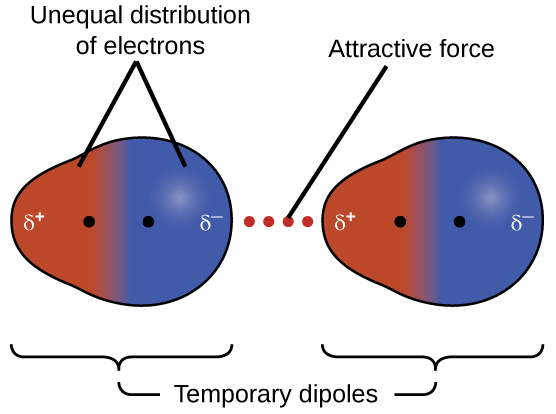
London dispersion Force
Always attractive
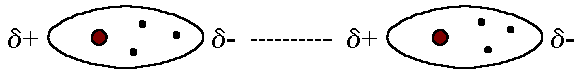
Dipole-Dipole Force
Attractive (stable) or Repulsive (unstable)
Hydrogen Bonding
A stronger dipole-dipole attracted
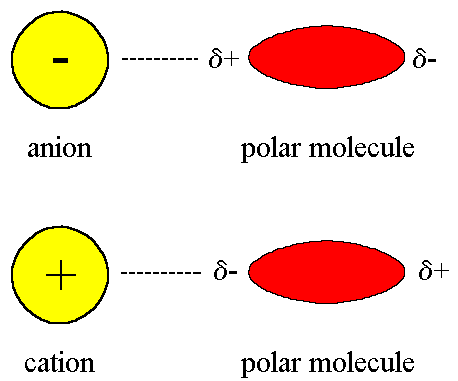
Ion-Dipole Force
Attraction between a ion (charged particle) and a polar molecules
Cation (+) attracted to a negative pole of a polar molecule
Anion (-) attracted to a positive pole of a polar molecule
Atomic Radius
The distance from the nucleus to the outermost electrons.
As you go down a group, the radius increases because the new electrons shells are added
As you across a period, the radius decreases because more protons pull the electrons in closer without adding a new shell
Ionization Energy (IE)
Energy required to remove an electron from an atom.
As you go down a group, it decreases because electrons are farther from the nucleus and are easier to remove
As you go across a period, Ionization energy increases because the nucleus pulls harder on the electrons.
Electronegativity
How strongly an atoms attracts electrons in a bond.
Increases across a period. (atoms want to fill their outer shell)
Decreases down a group (bigger atoms have a weaker pull on electrons).
Fluorine is the most electronegative element
Electron Affinity
The energy change when an atom gains an electron.
Generally, it becomes a more negative across a period (atoms want to gain electrons to complete their shells)
It becomes less negative down a group (larger atoms don’t pull new electrons as strongly)
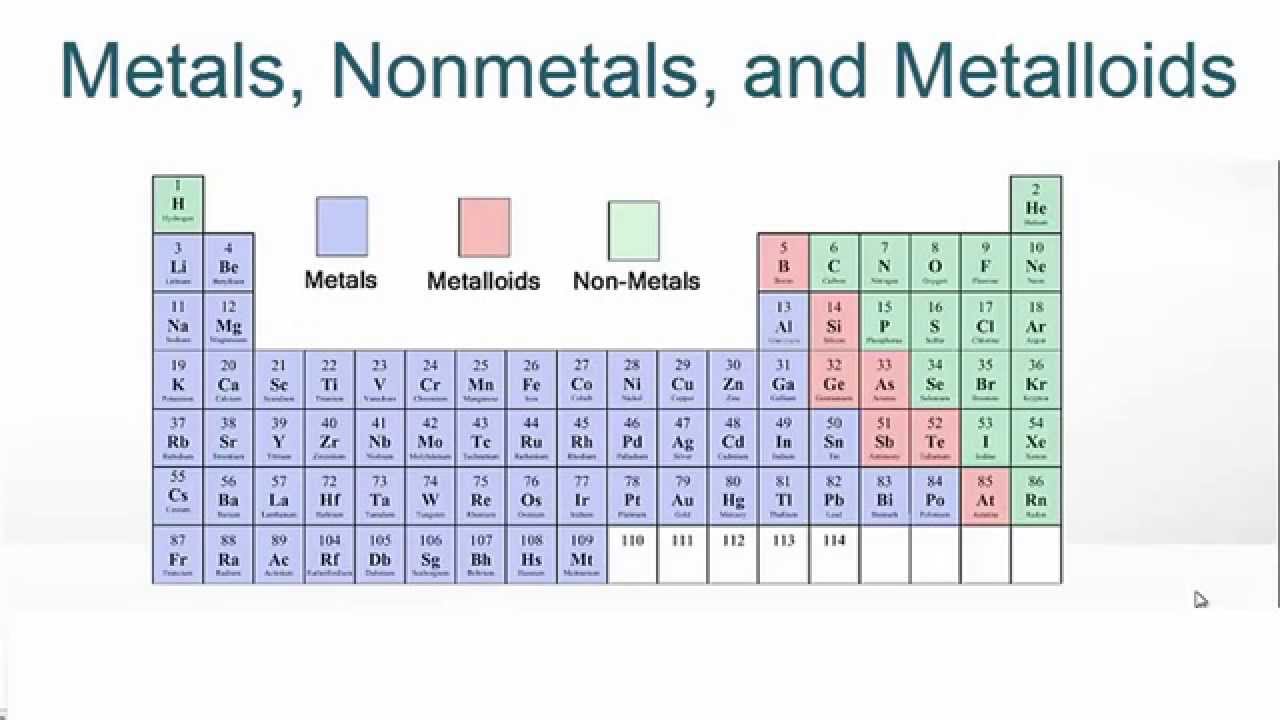
Metallic Character
How much an element behaves like a metal (shiny, conducts, loses electrons easily)
In increases down a group
decreases across a period
The bottom left of the table is the most metallic, and the top right is the least
Atom Sheiliding
When inner electron shells block the attraction between the nucleus and the outermost electron
Halogens
group 17 elements (F, CI, Br, I, Ar)
Very reactive non-metals that form salts with metals
Alkali Metals
Group 1 elements (Li, Na, K)
Extremely reactive metals especially with water
AKA Alkaline water
Noble gases
Group 18 elements (He, Ne Ar)
Very stable, nonreactive gases due to full valence electrons
Mendeleev
The secientist who created the first periodic table, arranging elements by atomic mass and leaving spaces for undiscovered ones
Transition metals
elements with partially dilled d sub-shell in its atoms or stable ion, found in groups 3-12 of the periodic table