Unit 2 Law FInal
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Explain how dextromethorphan is restricted in Wisconsin

Identify the authorized prescribers of prescription drugs in Wisconsin and explain their prescribing limitations, if any
Physician (DO and MD) | No Limits |
Dentistry | Limited to the oral cavity or its adjacent or associated tissues and structures or of the maxillofacial area and their impact on the human body |
Podiatrist | Limited to conditions affecting the foot and ankle |
Veterinarians | Cannot:
|
Optometrists |
|
Advanced Practice Nurse Practitioner (APNP) |
|
Physician Assistants |
|
List the requirements of a prescription order in Wisconsin and recognize when a required element is missing
Date of issue
First and last name and address of the practitioner
Name and quantity of drug product (dosage form and strength)
Directions for use of the drug product or device
Symptom or purpose for which the drug is being prescribed if the pt wants that
Refills
Practitioners written, electronic or digital signature
Name and address of the pt unless there is an exception per statute
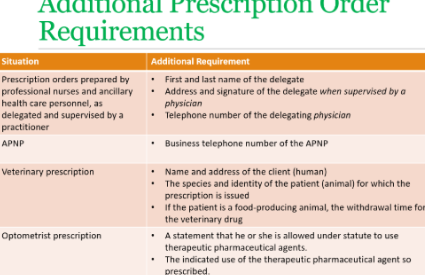
Explain when documentation of a prescription alteration must occur
Any alterations that modify the original intent of a prescription
Explain when a pharmacist may extend a prescription and recognize if a prescription may be extended in a given situation
Allowed | Prohibited |
ALL of the following: a) The pharmacist has been unsuccessful in attempting to procure a new prescription order or refill authorization for the drug after attempting to contact the prescribing practitioner or his or her office. b) The patient is on a consistent drug therapy program and the patient has previously refilled the prescription at that pharmacy or through another pharmacy in the same pharmacy chain. c) The drug is essential to the life of the patient, or the interruption of the drug therapy could result in undesirable consequences for the patient’s health. d) The pharmacist has not received and is not aware of written or oral instructions from the prescribing practitioner prohibiting further dispensing pursuant to or extension of the prescription order |
|
Determine if two drug products are therapeutic equivalents using the Orange Book or are interchangeable biologics using the Purple Book
Two drug products are therapeutic equivalents if they have the same two letter (+/- number) code in the Orange book and meet all other criteria of being therapeutic equivalents
Two biologic products are interchangeable if rated “I” in the purple book
Determine acceptable dispensing quantities for initial and refill prescription orders in Wisconsin
Unless a prescription specifies a medically necessary quantity, pharmacists can adjust initial and refill amounts based on professional judgment.
Adjustments can be made up to a 90-day supply in a single fill.
The initial fill must match the prescription unless the patient previously received that amount within the last 2 years
Identify the required elements of a prescription label in Wisconsin for drug products, biologics, compounded products, and veterinary products and be able to recognize if any required element is missing.
Drug products |
|
Veterinary Additional |
|
Compounded Drug Additional |
Sterile product label:
Sterile Product Labeling(Doesnt have to be on container)
|
Explain the PPPA requirements for prescription drugs and exemptions related to prescription drugs (including institutionalized patients)
Requirements | Exemptions |
The intent is to protect children from accidental poisonings with “household substances”, and by law, a food, drug, or cosmetic under FDCA falls in this category.
|
|
List the final check requirements in Wisconsin
DUR in WI
Compare and contrast the requirements for collecting and documenting patient information for OBRA ’90 and Wisconsin
Counseling Requirements: OBRA ’90 vs. Wisconsin
Category | OBRA ’90 | Wisconsin |
General Rule | Must offer to discuss medications with every patient or caregiver. | Counseling is required only in certain situations. |
When Required | Always offer counseling. | Required if: |
When Not Required | Patient can refuse the offer. | Not required if: |
Explain how to deliver prescriptions in accordance with US Postal Service and Wisconsin regulations
Mailing & Delivery of Prescription Medications
Only a pharmacist or medical practitioner may mail prescription medications.
Medications must be:
Securely packaged to prevent damage.
Shipped in a plain outer wrapper or packaging (no identifying pharmacy info visible).
Delivery Requirements
Delivery method:
Must use a common carrier or delivery service.
Delivery must be made to a location of the patient’s choice.
The method must be appropriate to prevent drug adulteration (i.e., no exposure to heat, moisture, etc.).
If a prescription is lost during delivery:
The pharmacy must replace it at no additional cost to the patient.
Pharmacy must provide a way for patients to report any delivery issues, including:
Delays or untimely delivery
Damaged medication upon arrival
Wrong or missing prescription drug or device
Explain the pharmacist and pharmacy’s responsibility regarding the duty to dispense contraceptives
● Pharmacist must deliver contraceptive drugs and devices to patient without delay unless:
○ Error or inadequate Rx order
○ Contraindicated
○ Potential fraud
● Fee if you violate this: $250–$2,500
● Abortion in WI
○ Legal, but prescriber may have to be present in room when drug is given
○ Cannot mail meds to patient in WI
Describe the requirements for dispensing prescriptions that are not patient-specific (opioid antagonists, epinephrine delivery systems, short-acting bronchodilators in schools, and glucagon in schools) and explain how these prescriptions are written differently

Recognize required elements of a Notice of Privacy Practices for a pharmacy

Explain when PHI may be fully disclosed, only the minimum disclosed, and not disclosed without authorization (including unique protections for psychotherapy notes and SUD records)
PHI Use & Disclosure Summary
Category | Details |
TPO (Treatment, Payment, Operations) | • PHI can be used/disclosed without patient authorization for treatment, payment, and operations purposes |
Fully Disclosed (No Authorization Needed) | • To patient or personal representative |
Minimum Necessary Disclosure | • Applies to all other situations not covered under TPO or listed exceptions |
Not Disclosed Without Authorization | • Psychotherapy notes (unless required by law) |
Do NOT Disclose | • If patient requests non-disclosure for an item/service they fully paid for out-of-pocket |
SUD (Substance Use Disorder) Notes | • Patient must give consent at least once for future TPO disclosures |
Incidental Disclosure | • PHI disclosed unintentionally |
List requirements of a chart order and identify how chart orders are different from outpatient prescription orders
The date of issue
The name of the practitioner
The name, strength, form, and quantity of the drug product or device prescribed
Directions for the use of the drug product or device
The symptom or purpose for which the drug is being prescribed if required (requested by patient to appear on the label)*
If the order is written by the practitioner, the signature of the practitioner (handwritten or electronic)
First and last name and address of the patient
Patient’s medical record number OR date of birth
If the order is prepared by a delegate of the practitioner, the first and last name and signature of the delegate (handwritten or electronic) AND the first and last name of the practitioner
No need for a practitioner address
Differentiate the requirements for emergency kits and contingency supplies in a nursing home
Emergency Medication Kits in Nursing Homes | Contingency Supply of Medications in Nursing Homes |
|
|
Explain how medications for nursing home residents are stored and packaged, including requirements for unit doses
Storage. Medications shall be stored near nurse's stations, in locked cabinets, closets, or rooms, conveniently located, well-lit, and kept at a temperature of no more than 85°F (29°C).
Transfer between containers. Medications shall be stored in their original containers and not transferred between containers, except by a physician or pharmacist.
(Missing from the list in the slide but possibly exists in the regulation.)
Separation of medications. Medications packaged for individual residents shall be kept physically separated.
Refrigeration. Medications requiring refrigeration shall be kept in a separate covered container and locked unless the refrigeration is available in a locked drug room.
External use of medications. Poisons and medications for external use only shall be kept in a locked cabinet and separate from other medications, except that time-released transdermal drug delivery systems, including nitroglycerin ointments, may be kept with internal medications.
Accessibility to drugs. Medications shall be accessible only to the registered nurse or designee. In facilities where no registered nurse is required, the medications shall be accessible only to the administrator or designee. The key shall be in the possession of the person on duty and assigned to administer the medications.
Define and differentiate the responsibilities of the central shared service pharmacy, labeling pharmacy, and originating pharmacy
central shared service pharmacy | "Central shared services pharmacy" means a pharmacy licensed in this state acting as an agent of an originating pharmacy. |
|
labeling pharmacy | "Labeling pharmacy" means the central shared services pharmacy or originating pharmacy which is responsible for product [and label] verification. |
|
originating pharmacy | "Originating pharmacy" means a pharmacy licensed in this state that uses a central shared services pharmacy. |
|
List the requirements to be a pharmacist delegate for a remote dispensing site
Be 18 years or older with at least a high school education
Have 1,500 hours of dispensing experience within the last three years or have completed an accredited pharmacy technician training program
Delegates may include:
Post-2nd-year pharmacy students
Out-of-state pharmacists seeking WI licensure
Registered pharmacy technicians
Pharmacy graduates
Explain when remote dispensing is not allowed to occur
The pharmacist is unavailable remotely
The patient or delegate cannot communicate with the pharmacist
List the qualifications to be a pharmacy product verification technician
(b) Completed an accredited pharmacy technician training program or has 500+ hours of experience in product selection, labeling, and packaging.
(c) Completed a didactic and practical training curriculum approved by the supervising pharmacist, including training in six key domains.
(d) Successfully completed a validation process.
(e) Individuals who completed the board’s pilot program (Oct 1, 2016 – Sept 30, 2019) qualify unless they fail to meet quality assurance standards.
Contrast who is responsible for a delivery system vs. an automated direct-to-patient dispensing system
A licensed pharmacy controls the delivery system, with a managing pharmacist responsible for policies.
A supervising practitioner ensures compliance for practitioner dispensing in healthcare facilities, offices/clinics, and correctional/rehab facilities.
Explain who can administer a vaccine in Wisconsin and the requirements these individuals must meet
For patients 6 years and older:
Pharmacists |
|
Pharmacy students & out-of-state pharmacists |
|
Pharmacy technicians |
|
For patients under 6 years:
Pharmacists |
|
Describe when a pharmacist must make a report to their local health department or DHS in Wisconsin
A pharmacist (or pharmacy) must make a report to their local health department when:
there is an unusual increase in prescriptions for antibiotics,
when a drug is dispensed for a relatively uncommon disease or may be associated with bioterrorism.
Describe the two ways a consumer could access a poison in Wisconsin
A consumer can access a poison through:
a prescription order,
the “labeled delivery” process. The “labeled delivery” process requires labeling, recordkeeping, informing the customer of the poisonous nature of the substance, and determining the poison will be used for a lawful purpose.
Identify the types of products and transactions included in DSCSA
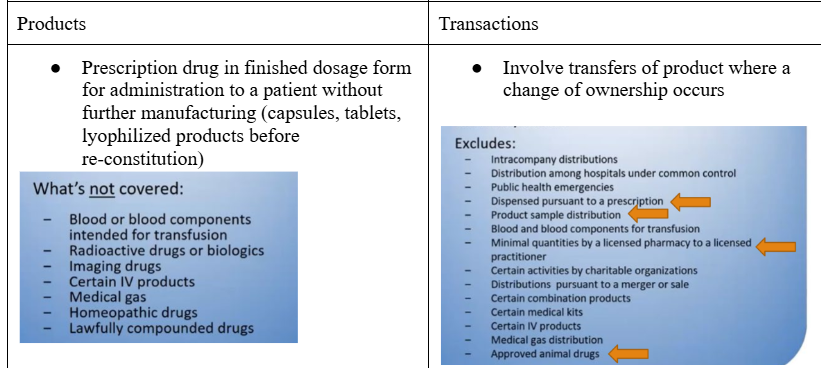
Explain the four responsibilities of a dispenser under DSCSA
1) Engage in business only with licensed, registered [authorized] trading partners: | a) Manufacturers and repackagers must have a current registration with the FDA b) Wholesale distributors and third-party logistics providers must be licensed (state or federal) c) Dispensers must be licensed (state) |
2) Properly manage product tracing documentation: | a) Only accept prescription pharmaceutical products if they arrive with transaction history (TH), transaction information (TI), and transaction statement (TS). * Will not be required/enforced when electronic track and trace recordkeeping begins. |
3) Implement a system and process to properly manage (investigate and handle) suspect and illegitimate prescription pharmaceutical products | a) Quarantine any suspect prescription pharmaceutical product(s). Note: Store-related documentation for 6 years after the conclusion of the investigation or disposition. |
4) Pharmacies must have policies and procedures in place that allow for unit-level traceability under DSCSA, including: | a) Ensuring that all required TI (Transaction Information) and TS (Transaction Statement) are exchanged via a secure, interoperable, electronic system. Exemptions:
|
Identify when a compounded drug violates FDCA 503A
Failure to meet any of the following:
1) Compounded drug must be for an individual patient pursuant to a valid prescription order and compounded by a licensed pharmacist or physician. | |
2) If the compounded drug is prepared in anticipation of receiving a prescription, a limited quantity is prepared: |
|
3) The compounded drug cannot essentially be a copy of a commercially available product, unless compounded occasionally and not in inordinate amounts |
|
4) It is compounded in compliance with USP chapters on compounding [USP <795> and <797>] and using bulk substances that comply with monograph standards, if one exists. |
Note: USP <795> and <797> have the force of federal law for compounding under the federal definition! |
5) The bulk drugs used for compounding are manufactured by an entity registered with the FDA. | 6) It is compounded with ingredients (other than bulk substances) that comply with USP standards. |
7) It does not include drugs from an FDA list of items withdrawn from the market due to safety or efficacy concerns. | 8) It does not include drug products identified by the FDA as presenting difficulties for compounding due to safety or efficacy risks |
9) The compounder does not distribute more than 5% of total prescriptions dispensed or distributed by the pharmacy unless an MOU exists between the pharmacy and the FDA. |
Describe how a 503B outsourcing facility is different from a 503A pharmacy
503B outsourcing facilities can compound sterile products in unlimited quantities without individual prescriptions, which 503A pharmacies cannot.
503B outsourcing facilities do not have to be licensed pharmacies, but compounding must be done by or under direct supervision of a pharmacist.
503B outsourcing facilities have less flexibility to compound drugs that are “essentially a copy” compared to 503A.
Identical copies cannot be compounded just for a clinical difference.
Non-identical copies with a meaningful change for the patient are allowed.
Identify if a repackaged product is compliant with FDA guidance
1) The drug being repackaged is an FDA-approved prescription drug or an unapproved drug on the drug shortage list. | 2) Repackaging happens in a state-licensed pharmacy, outsourcing facility, or federal facility. |
3) The drug is repackaged by or under the supervision of a licensed pharmacist. | 4) If repackaged in a state-licensed pharmacy or federal facility, distribution happens only after receiving a valid prescription for a specific patient. |
5) The drug is repackaged, stored, and shipped in a way that does not conflict with approved labeling (except for single-dose or single-use labeling changes). | 6) The container used for repackaging must be suitable for storage through the drug product’s BUD (Beyond-Use Date). |
7) Repackaged drug labeling must include the same storage and handling instructions as the approved drug product. | 8) The repackaged drug is assigned a BUD based on guidance, unless scientific data supports a shorter BUD. |
9) If repackaged in a state-licensed pharmacy or federal facility:
| 10) The repackaged drug cannot be on a list of drugs withdrawn from the market due to safety or efficacy concerns. |
11) A repackaged drug cannot be sold or transferred by any entity other than the one that repackaged it. | 12) Repackaged drugs can only be distributed in states where the repackaging facility meets all applicable state requirements. |
13) Drugs repackaged by an outsourcing facility must meet specific label and container requirements. |