Redox Recap & Half Equations
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What is oxidation?
Loss of electrons.
What is reduction?
Gain of electrons.
What acronym can be used to remember oxidation and reduction?
OILRIG
Oxidation Is Loss (of electrons).
Reduction Is Gain (of electrons).
What is an oxidising agent?
A species that oxidises another species by removing electrons from it. It therefore becomes reduced itself in the process.
What is a reducing agent?
A species that reduces another species by donating electrons to it. It therefore becomes oxidised itself in the process.
What is the oxidation number of all elements?
0.
What must the oxidation numbers of all atoms in a neutral molecule add up to?
They must add up to 0.
What must the oxidation number of all atoms in an ion add up to?
They must add up to the overall charge of the ion.
The more electronegative atom must have what oxidation state?
A negative oxidation state.
What are the characteristic oxidation states of some certain elements you need to know?
Group 1 metals always have an oxidation state of +1.
Group 2 metals always have an oxidation state of +2.
Hydrogen always has an oxidation state of +1 (except as part of a metal hydride, where it has an oxidation state of -1).
Fluorine always has an oxidation state of -1.
Oxygen always has an oxidation state of -2 (except in a peroxide where it has an oxidation state of -1, or in a compound with fluorine, where the oxidation state is positive).
Chlorine always has an oxidation state of -1 (except in a compound with oxygen or fluorine, where the oxidation state is positive).
How can you determine if a species is oxidised or reduced from the oxidation states?
If the oxidation state of an element in a given species becomes more positive, that species has been oxidised during the reaction.
If the oxidation state of an element in a given species becomes less positive, that species has been reduced during the reaction.
What is a half equation?
A full chemical equation can be split into 2 half equations; one showing the oxidation and one showing the reduction.
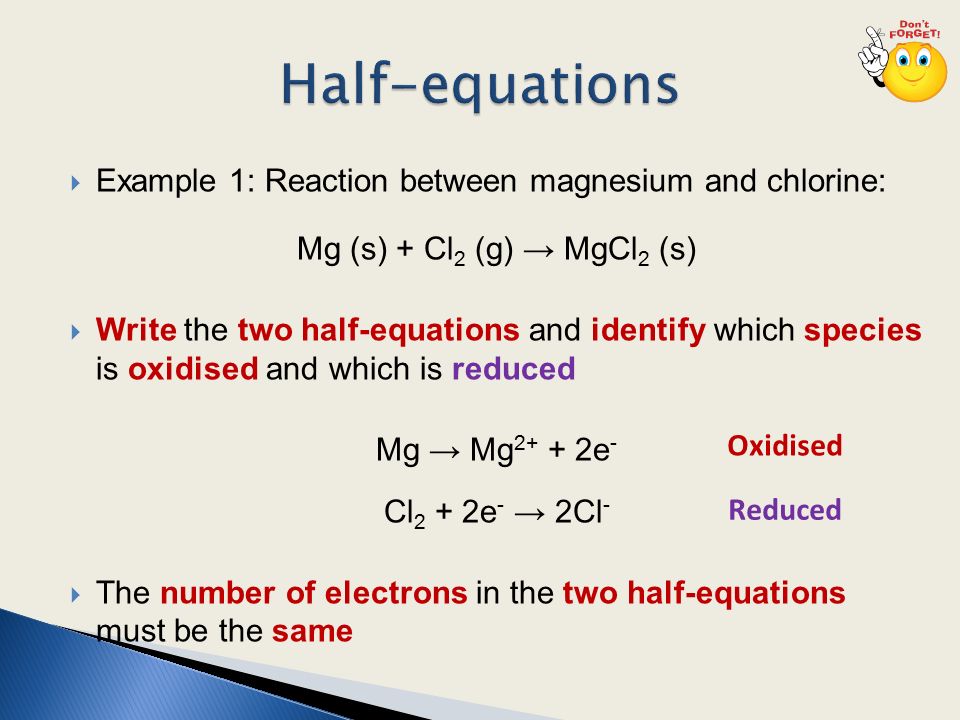
State the rules needed when writing a half equation.
Balance all species apart from oxygen and hydrogen.
Balance any oxygen with water (H20).
Balance any hydrogen with H+ ions.
Balance charges with electrons (e-).
Write a half equation showing the conversion of Cr2O72- to Cr3+.