Chemistry-energy changes and intermolecular forces
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Chemical reactions
Chemical reactions involve the rearrangement of atoms from one form to another. During the process, energy can be released from the chemicals or taken in by chemicals as they change from reactants to products. The energy can be released or taken in heat (most common), electricity or light.
Enthalpy
The energy chemicals have is chemical potential energy which is also known as enthalpy.
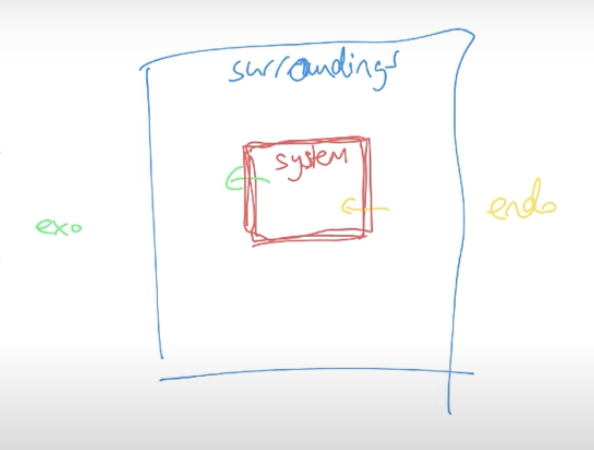
Systems and surroundings
The transfer of energy into and out of chemicals can be referred to in terms of the system and surrounding. The system is all the chemicals involved in the reaction. The surrounding is all the substances not involved in the reaction.
Endothermic reactions
In endothermic reactions, energy is taken from the surroundings and put in the system, thus their enthalpy increases.
Exothermic reactions
In exothermic reactions, energy is put into the surrounding from the system, and thus their enthalpy decreases.
Rearranging of particles
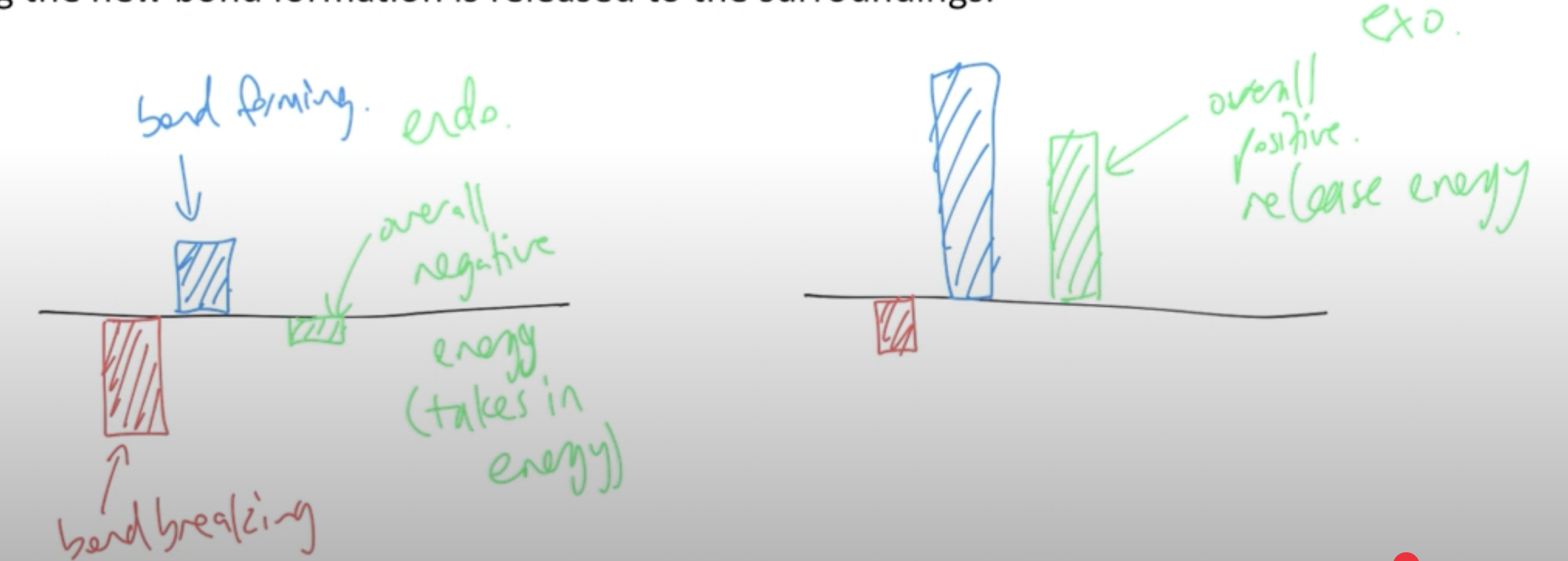
For both endothermic and exothermic reactions, both need to rearrange their particles. To rearrange particles first you have to break the bonds that hold the particles together. This requires energy. The stronger the bond, the more energy needed to break it. The second part of rearranging particles is the formation of new bonds with new particles. This releases energy. Like two hands clapping, when things come together, they snap together and release energy. The stronger the bond, the more energy is released.
How do the relative amounts of energy needed to break bonds and energy released when bonds are formed determine whether energy is absorbed or released by the reaction?
For reactions where the energy needed for bond breaking is high, but the energy released during bond formation is low, then the overall reaction is endothermic, where energy is collected by the system and held as enthalpy. For reactions where the energy needed for bond breaking is low but the energy released during bond formation is high, then the overall reaction is exothermic, where extra energy during the new bond formation is released to the surroundings.
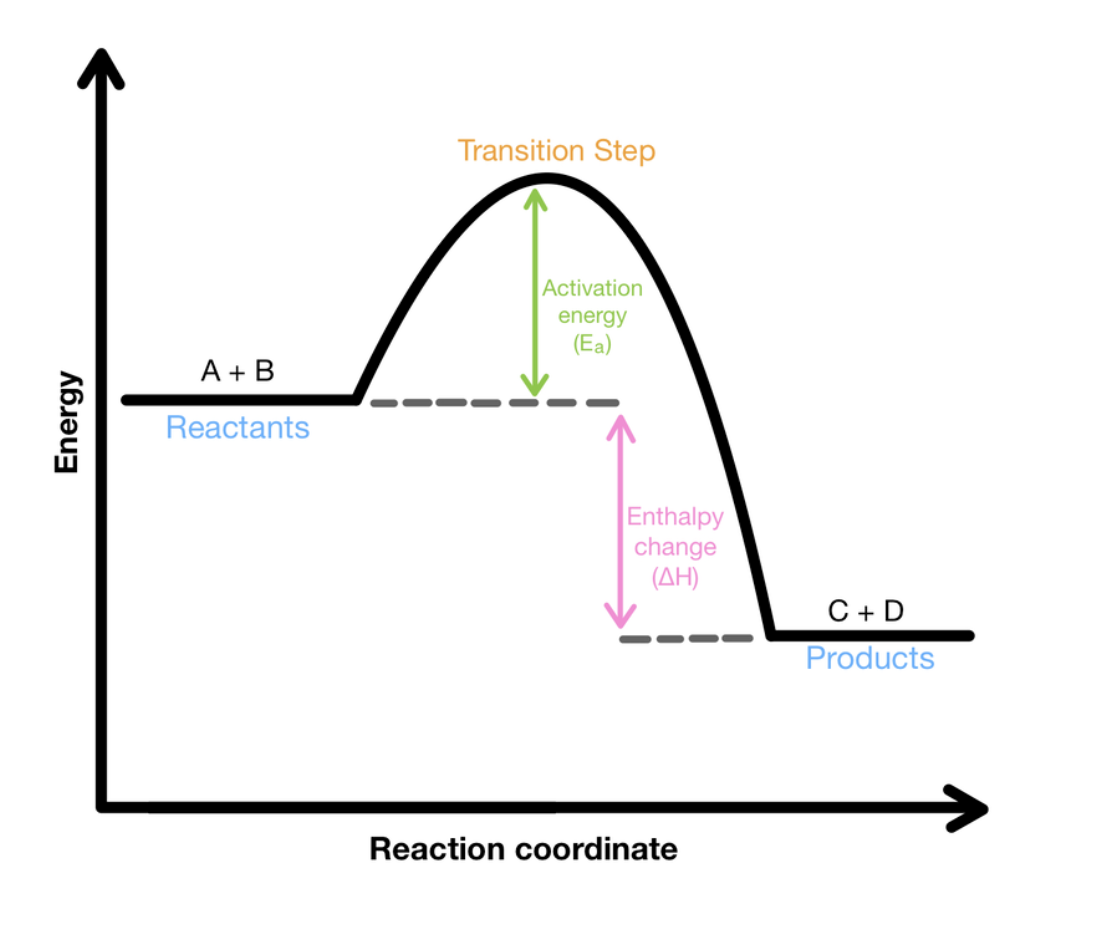
Activation energy
The amount of energy per mole that is required for the reaction to occur.
Transition state
The point in time when all particles have broken their bonds and not yet have formed new bonds is called the transition state. This is the point of highest enthalpy due to the fact that it has absorbed energy in, but not yet released it out yet.
Two Forms of Thermochemical Equations
Using ΔH (H=enthalpy, meaning enthalpy change) - in which a semicolon follows the balanced chemical equation, then a ΔH= [the amount of energy gained in the reaction]kJ. If it is positive, the substance has gained energy in the reaction and thus is endothermic, and if negative, it has lost energy and is exothermic. The value in kJ is the amount of energy gained per (mole X coefficient of each element individually) Within the equation - where the energy is shown to be on either the reactants side or the products side of the equation. If it is on the reactants side, it is endothermic (as the energy is taken in and used as a reactant), and if it is on the products, it is an exothermic reacton (as the energy is released seperate from the products) The value in kJ is the amount of energy transferred by coefficient number of moles of a substance (e.g., if there are 2O2s, then the value would be the amount transferred for every two moles of oxygen gas)
ΔH
Delta H, represents the change in enthalpy between reactants and products
Energy Profile Diagram
Graph which depicts the changes in enthalpy in a reaction as it transforms from reactants to products. Has no absolute values for the either axis as it is impossible to measure enthalpy, and a reaction occurs instantaneously meaning time cannot be measures. These changes in enthalpy only occur during a reaction, meaning the enthalpy of reactants and products will be constant.
Equation to find Energy Change from moles and ΔH and vise versa
E(A) = ΔH x n(A) ÷ CE[coefficient](A) n(A) = E(A) x CE(A) ÷ ΔH ΔH = E(A) x CE(A) ÷ n(A)
Valence Shell Electron Pair Repulsion Theory
The theory that molecules will take on specific shapes as the pairs of electrons of the atoms covalently bonded try to get as far away from one another as possible, whilst still being attracted to the neucleus/neuclei of the atom/s (if the electron is within the covalent bond)
How to Identify the Shape of a Molecule
Must first identify both the number of regions of negative space, and the number of non-bonding pairs of electrons around the central atom of a molecule (atom bonded to all others or all sidechains), then use following chart: [chart thing you know what im talking about]
The Two Ways a Bond in a Molecule Can be Classed
Polar
Non-Polar
Polar Bond
A covalent bond between two atoms of different electronegativities, where the electrons are not shared evenly between atoms as they are attracted closer to the one of a greater electronegativity, thus creating a positive and negative end to the bond as the electrons spend a majority of the time closer to one atom. The greater the difference in negativities between atoms, the greater the polarity of the bond
Non-Polar bond
A covalent bond between two atoms of the same element, thus having equal electronegativities, thereforce resulting in the electrons within the covalent bond staying in the centre, as neither atom can pull the electrons harder than the other. This means that neither side of the bond is more or less positively or negatively charged than the other
Bond between N, O, or F and Hydrogen
An extremely strong polar bond due to the great difference in electronegativities between elements, meaning that the N, O, or F pulls on the shared pair of electrons particularly closely. This causes the N/O/F to be extremely negatively charged, whilst the hydrogen becomes extremely positively charged
Polar Molecule
A molecule which has an uneven distribution of electrons, due to both polar bonds and the shape of the molecule, causing the centres of positive and negative charge to be in different place, creating a positive and negative side to the molecule. Must be determined by considering the symmetry and presence of non-bonding electrons within a molecule.
Symmetry for Molecules
The state of the positive and negative centres of a 3D molecule being in the same place, making a non-polar molecule
Seperating Molecules vs Separating Atoms within Molecule
Seperating Molecules, as in the case of boiling or melting, does not require a relatively large amount of energy due to weak intermolecular forces. However, due to the strength of intramolecular forces, seperating atoms in a molecule DOES requires a relatively large amount of energy.
Temporary Instantaneous Dipole
The dipole occurs when the electrons in a particle, which are in constant random movement, go to positions in which the negative charge is unevenly distributed across the particle, creating a positive and negative end to the particle. This lasts for an extremely short amount of time, hence 'instantaneous'.
Dispersion Forces
The attraction between the positive end of one particle and the negative end of another due to temporary instantaneous dipoles. This occurs as when a T.I.D occurs in one particle, the electrons on the negative end will repel the electrons of neighbouring particles, creating more T.I.Ds, thereby creating dispersion forces. Increase as electrons increase, as more electrons creates more frequent T.I.Ds of greater strengths. Generally, molecular mass is proportional to the number of electrons, and thus can be used to compare dispersion forces is that is all that is available.
Dipole-Dipole Forces
The attraction between the negative and positive ends of two polar molecules. Polar molecules have two permanent dipoles due to their structure having unevenly distributed electrons, and when they attract to many other polar molecules, will align in a lattice that arranges oppositely charges ends next to each other. These forces increase with the difference in electronegativities between atoms in the molecule, as well as vary with the shape and arrangement of the molecule.
Hydrogen Bond
"The extremely strong bond that arises between a hydrogen bonded to an N/O/F and the lone pair of electrons of another N/O/F, occuring do to the great difference in electronegativities between N, O and F, and hydrogen, allowing for the hydrogen to bond to N/O/F's in neighbouring molecules due to it's extreme positive charge. Does not vary in strength as in the case of the other intermolecule forces, instead only being able to ""increase"" in a substance with it's ability to form more hydrogen bonds (i.e., more hydrogens/N/O/Fs)"
Sum of Intermolecular Forces
The sum of the contributions of each Van der Waals Force, dispersion, dipole-dipole, and h-bonds, to the total amount of force between molecules. The strength of this determines the melting/boiling point of a substance, as the stronger the bonds between molecules, the more heat energy is required to break these bonds and change the state of matter of a substance. Each Van der Waals Force can contribute different amounts, hydrogen bonds do not always contribute the most and dispersion forces do not always contribute the least
Vapour Pressure
The amount of pressure that is exerted on the walls of a sealed container by the gaseous particles of a substance evaporated when a liquid is put at rest. The amount of pressure exerted is relative to the amount of particles evaporated from the liquid and converted to a gaseous state, which is in turn relative to the evaporation rate. This evaporation rate is dependent on the strength of bonds between particles, the SIMF, as the lower the SIMF, the more particles there will be at a sufficient energy to convert to a gaseous state. The higher the SIMF, the lower the evaporation rate, the lower the particles evaporated, the lower the vapour pressure.
How to Predict/Compare Physical Properties of Different Covalent Molecular Substances
Identify the Van der Waals Forces Present
Identify the substance with the higher SIMF with justification
Relate SIMF to property asked about. Should always refer to energy and bonds between particles