Unit 2 Honors Nuclear chemistry and the atom Deck 1
1/22
Earn XP
Description and Tags
Guaranteed 100%
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
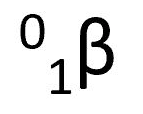
positron
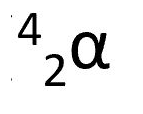
alpha particle
[...] radiation includes things like alpha particles and beta particles and is dangerous because it causes atoms to loses electrons
Ionizing
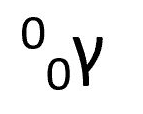
gamma particle
Release of nuclear particle which is shown on product side
Decay or emission
[...] radiation includes things like radiowaves and visible light and does not affect the atom
Non-ionizing
Refers to a nuclear particle being placed on reactant side with electron capture being an example
absorption
Electrons exist in the electron cloud which is also known as a [...] or [...]
shell; energy level
A [...] has an unstable nucleus and emits radiation to become more stable
radioactive isotope
What are two things that Rutherford discovered about the atom
Most of the atom is empty space and the nucleus is positively charged
Isotopes are atoms that have the same number of protons and a different number of neutrons which leads to an atom having a different [...].
mass
Two types of nuclear reactions are [...] where two atoms combine to form a bigger atom and [...] where a heavy nucleus is split into two smaller nuclei.
fusion; fission
Isotopes are atoms that have the same number of [...] and a different number of [...] which leads to an atom having a different mass.
neutrons; protons
Which subatomic particle(s) mass is 1 amu?
proton and neutron
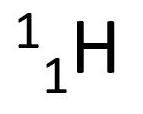
proton
The time required for half of the atoms in any given quantity of a radioactive isotope to decay
half life
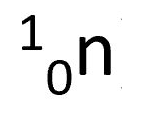
neutron
The majority of the mass of the atom is located inside the [...] which is composed of [...] and [...]
nucleus; protons; neutrons
Illness or sickness caused by excessive exposure to radiation
Radiation poisoning
The three subatomic particles are [...] which have a positve charge, [...] which have a neutral charge and [...] which have a negative charge.
protons; neutrons; electrons
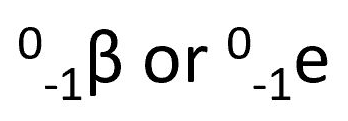
beta particle
[...] discovered the electron and came up with the [...] model of the atom
JJ Thompson; plum pudding
Coversion of one element to another through artificial means
transmutation