Suspensions - AK
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
What is a pharmaceutical suspension?
A heterogeneous system where solid drug particles (dispersed phase, typically 0.1–10 µm) are suspended in a liquid vehicle (continuous phase, usually water). Unlike solutions, suspensions are optically cloudy due to light scattering by particles.
How do suspensions differ from solutions?
Solutions: Homogeneous, molecular dispersion; optically clear; no sedimentation.
Suspensions: Heterogeneous, particulate dispersion; cloudy; prone to sedimentation.
Why are suspensions used in pharmaceuticals?
or drugs with low aqueous solubility where solution formulations are impractical. Common routes: oral, topical, ophthalmic, parenteral (e.g., intramuscular).
What is the electrical double layer (EDL)?
A charged layer around particles in water:
Fixed (Stern) layer: Tightly bound counterions (e.g., H⁺ from H₃O⁺).
Diffuse (Gouy-Chapman) layer: Loosely bound counterions (e.g., Na⁺).
What is a zeta potential?
Potential at the shear plane between these layers; indicates stability.
How does pH affect particle charge?
Hydrophobic particles acquire negative charge in water due to OH⁻ adsorption (from H₂O autoionization). pH adjustments alter ionization of surface groups.
What factors affect the EDL thickness?
Ionic strength: High ionic strength (e.g., NaCl) compresses the diffuse layer (↓ Debye length, 1/κ).
Surfactants: Adsorb to particle surfaces, altering surface potential (ψ₀).
What does DLVO theory predict?
The balance between:
Van der Waals attraction (Vₐ): Always negative (promotes aggregation).
Electrostatic repulsion (Vᵣ): Positive (stabilizes suspension).
Total interaction energy (Vₜ) = Vₐ + Vᵣ.
Describe the three interaction zones:
Primary minimum: Deep energy well → irreversible coagulation (bad).
Primary maximum: Energy barrier → deflocculation (risky if kinetic energy overcomes barrier).
Secondary minimum: Shallow well → reversible flocculation (ideal for suspensions).
How do additives impact DLVO behavior?
Ionic excipients (e.g., NaCl): Compress EDL → deeper secondary minimum (promotes flocculation).
Surfactants: Modify ψ₀ → may stabilize or destabilize.
What governs particle movement in suspensions?
Diffusion (Brownian motion): Dominant for particles <1 µm (per Stokes-Einstein equation).
Sedimentation (Stokes’ law): Dominant for particles >0.5 µm.
What is the sedimentation volume ratio (F)?
F=Vf/V0, where:
VfVf: Final sediment volume.
V0V0: Initial suspension volume.
Flocculated systems: High F (~0.6), loose sediment.
Deflocculated systems: Low F (~0.1), dense "cake."
How to control sedimentation?
Reduce particle size (↓ r in Stokes’ law).
Increase medium viscosity (↑ η, e.g., with polymers like HPMC).
Match densities of particle and medium (e.g., add dextrose).
What is Ostwald ripening?
Growth of large particles at the expense of small ones due to temperature cycling:
High temp: ↑ solubility → small particles dissolve.
Low temp: ↓ solubility → drug precipitates on large particles.
Solution: Use drugs with flat solubility-temperature profiles.
How does wetting affect stability?
Hydrophobic particles resist wetting → clump. Surfactants (below CMC) reduce interfacial tension → improve dispersion.
Why is flocculation preferred?
Flocculated systems:
Sediment rapidly but are easily redispersed.
Avoid irreversible caking (deflocculated systems).
List excipients and their roles:
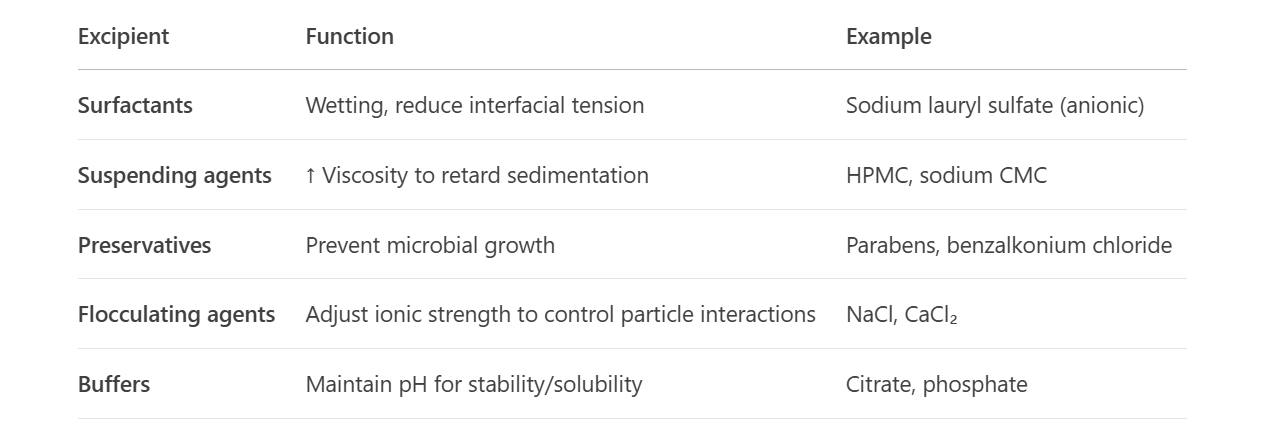
How does sodium CMC affect suspensions?
↑ Viscosity → slows sedimentation.
Releases Na⁺ → compresses EDL → may promote flocculation.
What are Key manufacturing challenges?
Initial dispersion: Avoid caking (use high-shear mixing).
Scale-up: Maintain uniform particle distribution.
Packaging: Stirred hoppers to prevent settling during filling.
How to assess suspension stability?
Sedimentation rate: Measure FF over time.
Redispersibility: Shake and check uniformity.
Particle size analysis: Ensure no Ostwald ripening.
What are the Requirements for oral suspensions?
Palatable (flavors/sweeteners).
Viscosity balance: Easy to pour but resists sedimentation.
What are the Requirements for ophthalmic suspensions?
Sterile (aseptic preparation; cannot be filtered).
Isotonic (~300 mOsm/kg), pH ~7.4.
Particle size <10 µm to avoid irritation.
How does particle size affect dissolution?
Smaller particles ↑ surface area (per Noyes-Whitney equation) → faster dissolution → better bioavailability.
What is the Hamaker constant (A)?
Material-specific constant in VA=−Ar/12HVA=−Ar/12H. Reflects van der Waals attraction between particles in a medium.
Why avoid surfactants above CMC?
Micelles solubilize drug → converts suspension into a colloidal dispersion, altering stability.
A suspension shows irreversible caking. Possible causes?
Deflocculation: Particles in primary minimum.
Inadequate surfactant: Poor wetting → aggregation.
High ionic strength: Excessive EDL compression.
How to fix Ostwald ripening?
Use a drug with minimal solubility-temperature dependence.
Add polymeric stabilizers (e.g., PVP) to inhibit crystal growth.