Variable oxidation states
0.0(0)
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
1
New cards
Vanadium is formed how?
By the reduction of vanadate(V) ions by zinc in an acidic solution
2
New cards
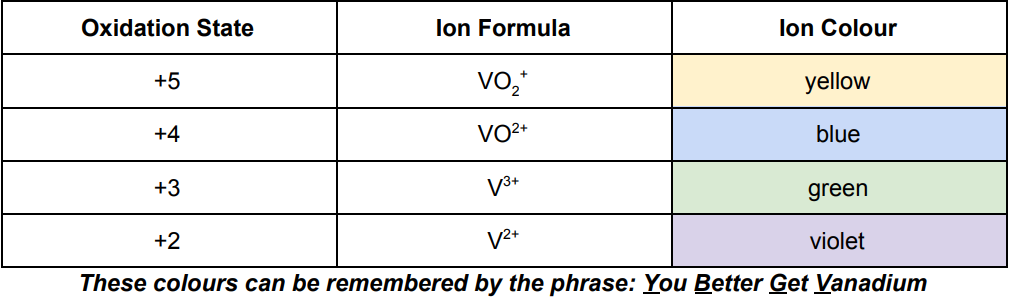
Vanadium all oxidation states
3
New cards
Vanadium reduction reaction
In acidic conditions:
2VO2 + + 2e- + 4H+ → 2VO 2+ +2H2O
In alkaline conditions:
VO 2+ + H2O → VO2 + + 2H+ + e-
4
New cards
Tollen’s reagent
Silver complex [Ag(NH3)2]+ is reduced by aldehydes to form silver atoms
![<ul><li><p>Silver complex [Ag(NH3)2]+ is reduced by aldehydes to form silver atoms</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/5f0bf2bf-f9bd-4f24-aae1-f438d5c977d3.png)
5
New cards
Redox titrations of Fe(II), C2O4 2- and MnO4-