PMI 128 Midterm 1
1/201
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
202 Terms
Bacteriophage weight
10^-15 grams
Bacteriophage
A virus that infects bacteria
what percent of nucleic acid in plasma is virus?
94% -pooled plasma samples from 120 healthy donors in Spain to high-speed centrifugation, RNA and DNA extraction, random amplification, and massive parallel sequencing.
what is a virus
very small, infectious, obligate intracellular parasite - w/ DNA or rna genome :
in permissive cells, the viral genome replicates and uses the cellular systems to produce progeny virions. (molecular parasite)
• progeny virions are assembled from components synthesized de novo in a host cell.•
progeny virions transmit the infection to next host cell or organism
Viron
physical virus particle
Virus
any stage of the life cycle
viral diseases
manifestation of the infection
difference between the virus and cellular empire
cells encode ribosomes while viruses only encode capsids to code the outside of their genome
viral empire domains
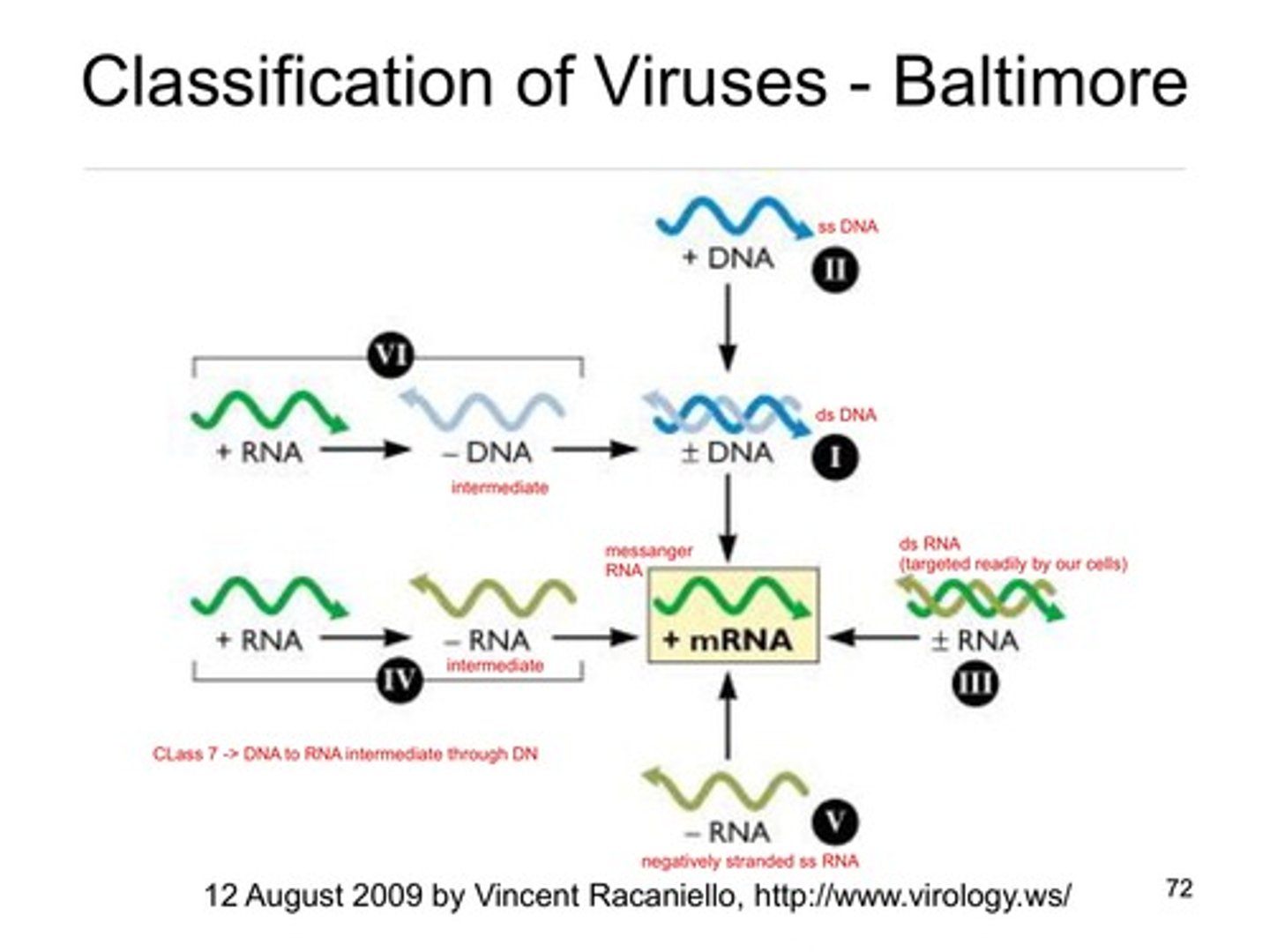
+R: positive-strand RNA viruses
−R: negative-strand RNA viruses
dsR: double-stranded RNA viruses
dsD: double-stranded DNA viruses
ssD: single-stranded DNA viruses
RT: retro-transcribing elements/viruses
VR: viroids
How do viruses replication
-require a host cell
-Transcribed into mRNA that can be replicated by host ribosomes
-Need host overall to conduct protein synthesis, make membranes, and generate energy
Polymerases
replicate virus genomes
capsid proteins
Capsid proteins that package genomes to form virions (''structural VHPs'')
Viral Hallmark proteins
VHPs have only distant homologs among cellular proteins, suggesting ancient origins -Capsids and Polymerases that replicate viral genome
small pox virus facts
limited host range
20-40% mortality
80-90% morbidity (sickness)
life long immunity
VARIOLATION WAS THE FIRST ATTEMPT AT IMMUNIZATION
innoculation
injecting a person with a small dose of a virus to help build up a defense to disease -caused severe skin lesions at the site of inoculation and has a 1-2% fatality rate
Influenza virus origin
Broad host range: humans, pigs, chickens, ducks, whales etc...•
Influenza is thought to have arisen as a human disease as a consequence of cross-species transmission from pigs and chickens to human when animals were domesticated about 6,000 ago.
HIV facts
limited host range: humans, chimpanzees
• 100% mortality
• HIV is thought to have arisen as a human disease as a consequence of cross-species transmission from chimps to humans when animals are hunted and butchered.
• 40 million people are thought to be infected• In some African cities, 40-50% of reproductive-age adults are infected.
cowpox smallpox vaccine
• Immunized humans with vesicular fluids from human cowpox lesions and demonstrated protection from smallpox (human experiments)
.• First rationale human vaccine studies. used an attenuated (relatively weak) virus as the vaccine. coined the terms "vaccine" and"vaccination".
Cowpox as a smallpox vaccine
Vaccinia virus
Virus used in smallpox vaccination.
Germ Theory
the theory that infectious diseases are caused by certain microbes --determined that specific organisms are associates with processes (fermentation).
•conducted human vaccine studies(rabies). In 1885, Pasteur immunized human (Joseph Meister) with his attenuated rabies vaccine. (deliberate attenuation of a pathogenic virus).
Koch's postulates
To identify the agent responsible for a particular disease:
1) The organism must be regularly associated with the disease and its lesions
2) The organism must be isolated from the diseased host and grown in pure culture
3) The disease must be reproduced when a pure culture of the organism is introduced into a healthy, susceptible host.
4) The same organism must again be re-isolated from the experimentally infected host.
Koch's Postulates for the "Metagenomic" era
To identify the agent responsible for a particular disease using nucleic acid sequencing and bioinformatics:
1) A specific gene or genome must be able to uniquely distinguish samples from diseased subjects and healthy matched controls
2) The disease must be reproduced when a healthy subject is inoculated with a sample from a sick individual.
3) The specific gene or genome must be detected in samples from the experimentally infected host.
Taxonomic classification of viruses
1. Nature of nucleic acid in the virion
2. Symmetry of capsid
3. Presence or absence of an envelope
4. Size of virion and capsid
Hepadenoviruses
DNA ---> RNA ----> DNA
Retroviruses
Rna ---> DNA----> RNA -how virus replicates determines life cycle
Baltimore classification
How mrna is made
When did rna arise
4.4 billion years
Origin of Life on Earth
RNA to rna and peptides to protein to dna
Evolution of RNA virus timeline in relation to cells
RNA viruses evolved at the same time as precellular elements
exaptation
a trait that evolved for one purpose but later became useful for a different function
steps of virus evolution and capsid proteins
-exaptation of protocapsid genes by selfish replicators (replicons) from primitive cells
-recruited helicases and proteases to create an efficient replication process
-recruit dna metabolism and replication genes to expand genome and form a defense mechanism through exaptation of different viral and dna genes
Summary: how did viruses evolve
Viruses appear to have evolved from capsidless selfish genetic elements, and vice versa, on multiple occasions during evolution. At the earliest, precellular stage of life's evolution, capsidless genetic parasites most likely emerged first and subsequently gave rise to different classes of viruses (recruited capsid genes)
In vivo culture methods
1. Live animal inoculation
2. Bird Embryo ( pocks )
-inject the virus into the different spaces of the egg and let it grow for a couple of days - 10^11 infectious virons per chicken
-have to extract virus before the chick dies
in vitro culture methods
Tissue cultures, primary cell cultures, Diploid cell strains, and permanent cell line (transformed and primary), cytopathic effects
primary cells
cells that can only divide a limited number of times in-vitro (cells will either enter a senescent stage, or die)
immortalized cells
cells that can divide indefinitely in vitro
• growth factor-dependent
• sensitive to growth inhibitors
Transformed cells
have increased growth, loss of contact inhibition, tumor-specific transplant antigens, and T antigens, evade apoptosis, unlimited proliferation
outcomes of an in vitro infection
• Lytic infection
• Persistent infection
• Transforming infection
• Latent infection: no virus production
lytic infection
process in which a virus enters a cell, makes a copy of itself, and causes the cell to burst
persistent infection
slow release of virus without cell death
latent infection
Persistent infection with recurrent symptoms that "come and go"
transforming infection
The virus affects the host's genetic makeup, causing some kind of mutation in the host chromosome
cytopathic effect (CPE)
Morphologic evidence of cell damage and death caused by a virus replicating in a cell
CPE EXAMPLES
Cell swelling, detachment from culture dish, fusion of cells, inclusion bodies (accumulation of visions in cell)
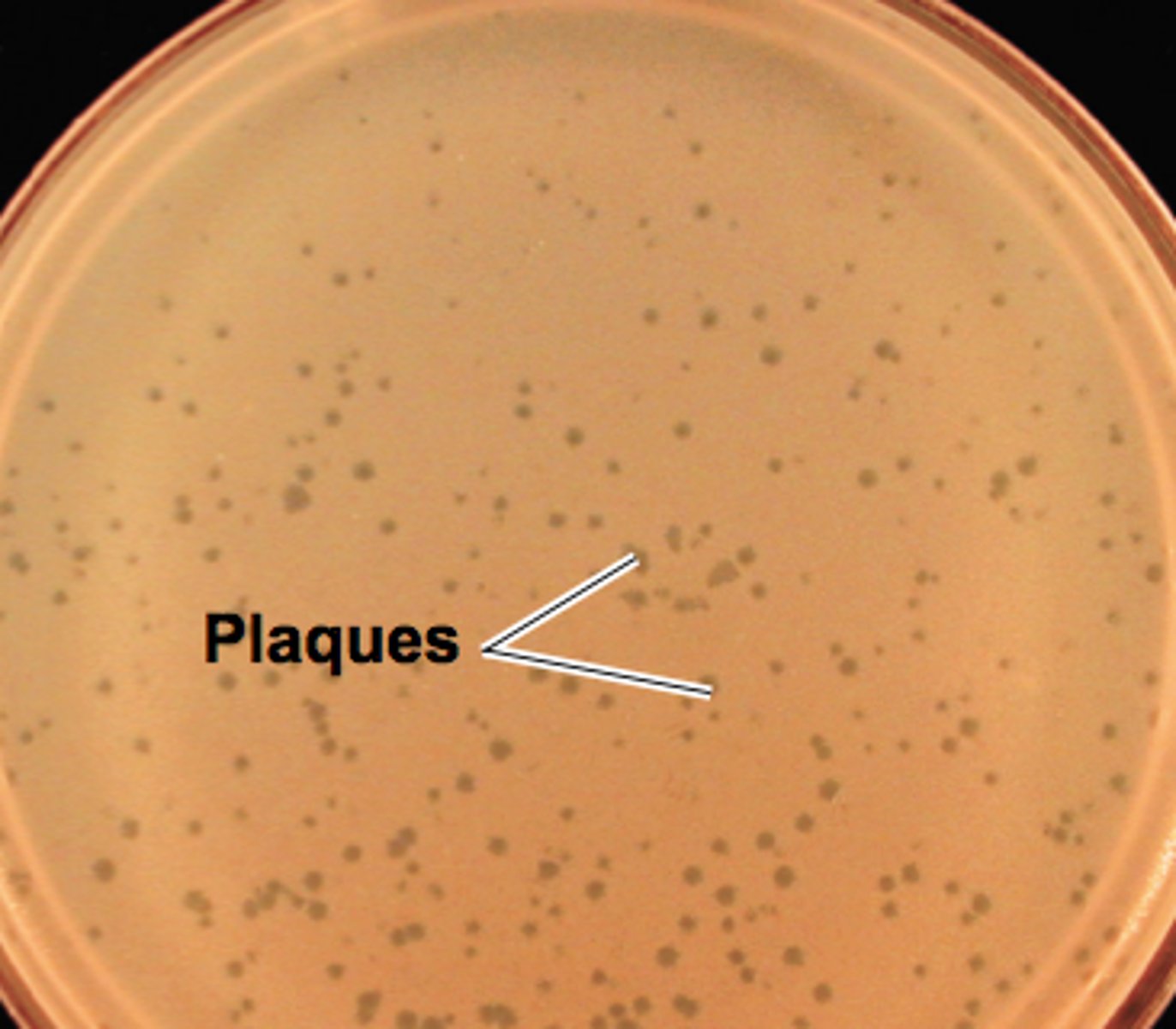
Viral Plaques
- areas on a petri dish full of bacteria where the bacteria have been killed off by the virus
- "clear zones"
- can be used to count viruses
eclipse period
What is the term of the viral growth period when no viruses can be found intracellularly?
When all cells are infected
there is one burst and then virus amounts level off
when a few cells are infected
there is a first burst, they infect other cells and then there is a second burst of cell -virus amount changes time and amount reproduced
EM method
count virons
ELISA
assay to quantify viral protein
PCR
assay to quantify viral nucleic acids
RT assay
quantifies viral enzyme activity
HAI
assay to bind to agglutinate particles -method 2-5 shows direct relationship between level of analyte and virion
plaque assay
# of plaques= number of infectious virons in the sample - more virions the more linear the amount of plaques -want to dilute stock to provide 10-12 plaques per petri dish
endpoint dilution assay cell suspension + CPE
some viruses do not make plaques - do a suspension assay in wells and then place under microscope to look for cell composition change -when half of the suspension wells are positive and the others are negative that is the 50 % infection point. Helps determine virus stock
Mortality ratio
The number of deaths per thousand people in a population /cells depends on viral stock dilution. Can determine how lethal a virus is
particle to PFU ratio
Number of virions /number of infectious virions in sample
• There is a high particle-to-PFU ratio for many animal viruses... so not all virions are infectious due to incomplete assembly, lethal mutations in genome, etc.
virion structure overview
contains @ least 1 protein coat, the capsid or nucleocapsid, which encases and protects the nucleic acid genome to form virions
Capsids
protein coats that enclose and protect the viruses nucleic acid
Construction of capsids from a small number of subunits minimizes the genetic cost of encoding structural proteins.
• Thus, capsids are assembled from identical copies of a small number of viral proteins with properties that permit regular and repetitive interactions among them -CAPSIDS ARE VHPS
Nucelocapsid
the capsid together with the nucleic acid
functions of the capsid
-protect genome
-bind target cell receptor in non envelope viruses
-introduce genome into the cell
polio symmetry
icosahedral capsid symmetry
rabies symmetry
helical capsid symmetry
virus life cycle
1. receptor-mediated attachment to the host cell surface
2. entry of viral genome into host cell
3. genome replication and mrna production in host cell
4. exit of progeny virus from host cell
plasma membrane role in virus entry
viral entry by fusion, viral exit from the cell, source of viral membranes
nuclear membrane in role of virus entry
mediates exit and entry of the virus into the nucleus for replication- DNA reproduces in here but rna replicates in the cytoplasm
lipid rafts
lipid-rich section of the plasma membrane used in virus assembly
Endosomes in viral replication
the virus undergoes acidification in endosome to change protein conformations and enter the cell.
vacoles in virus entry
how the virus is taken into the cell
ER in virus assembly
transport, protein folding, glycosylation and protein processing - source of membrane for viruses
Glycosylation
Addition of a carbohydrate group to a molecule.
what is a permissive cell?
Cells are permissive for viral replication if they contain the intracellular components required to support the biochemistry of replication.
what is a susceptible cell
Cells are susceptible to viral infection if they possess the specific cell surface molecule that serves as the viral receptor -JUST CUZ SUSCEPTIBLE DOES NOT MAKE PERMISSIVE
How do non-susceptible permissive cells become permissive?
made susceptible to a specific virus after transfection with, and expression of, the gene expressing the viral receptor
How do non-enveloped viruses interact with their cellular receptor
-capsid proteins (one or multiple)
-canyons or loops (polio)
- protruding fibers (adenovirus)
How do non-enveloped viruses interact with their cellular receptor?
Enveloped viruses interact with their cellular receptor via one or more of the surface glycoproteins in their host cell-derived membrane.
Receptor-mediated attachment to host cell - all steps may not be needed
1st step; accessory receptor binding- low affinity (may not be required, but increases efficiency of entry)
• 2nd step; primary receptor binding- high affinity (required for entry)
• 3rd step; co-receptor binding (required by some viruses)
CAR receptor
Coxsackievirus and adenovirus receptor; (member of the Ig superfamily)
CD4 receptor
cluster of differentiation antigen 4; (member of the Ig superfamily) -HIV-1
CD155
cluster of differentiation antigen 55; (member of the Ig superfamily) -poliovirus
CCR5
CCR5, chemokine receptor type 5; -HIV-1
CXCR4
• CXCR4, chemokine receptor type 4; -HIV-1
DC-SIGN
dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin; -HUMAN HERPES VIRUS 8
galNAc
N-acetylgalactosamine
GlcNAc
N-acetylglucosamine;
GM1,
monosialotetrahexosyl. -SV40
integrins
adenovirus, foot and mouth disease
Heparan sulfate proteoglycan
herpes simplex virus
sialic acid
influenza virus
HIV-1 receptor binding example
-The HIV 1 envelope glycoprotein mediates all interactions with target cell surface molecules.
1. Electrostatic interactions with heparan sulfate proteoglycans (HSPGs) can enhance the initial attachment of the virus particle for some strains (but can inhibit others). HSPG binding is not required for entry
2. The primary receptor for human immunodeficiency virus type 1 is CD4. Interaction with CD4 allow the envelope protein to engage a coreceptor, usually CCR5.
3. Binding to CD4 is required for binding to CCR5; hence CCR5 is a coreceptor.
4. Interaction with CCR5 induces further changes in the envelope glycoprotein that result in fusion of the viral and target cell membranes. Examples of virus attachment factors, receptors, and co-receptors.
Sialic acid and protein receptor binding events during CoV infection
1. CoV spike proteins mediate weak interactions with abundant host surface sialates, keeping viruses concentrated on cells yet potentially diffusible across plasma membranes.
2. S proteins subsequently engage protein receptors and are proteolytically activated into membrane fusion-inducing conformations
Multiple receptors for herpes simplex virus 1 (HSV-1).
Six (of 15) viral surface glycoproteins are shown, four of which are essential for entry.
1. Initial attachment to HSPG (heparan sulfate glycosaminoglycans) is mediated by gC and gB.
2. gD engages the main receptor, which can be either nectin-1, HVEM (herpesvirus entry mediator), or 3-OS-HS (3-O-sulfated heparan sulfate).
3. Subsequently, gD binds the gH/gI heterodimer, which then activates gB to mediate fusion.
4. The entry route can differ depending on the cell. For example, the gK protein enables fusion at the plasma membrane of neurons. Multiple receptors for herpes simplex virus 1 (HSV-1).
what is special about viral receptors in cellular evolution
Virus receptors are cellular molecules (usually proteins) that have key roles in normal cell physiology. -can never evolve out
Cell surface molecules contribute to cell-cell adhesion and attachment to the extracellular matrix mainly composed of glycosaminoglycans (purple), fibrin (yellow), and collagen(blue). Tight junctions are formed by transmembrane proteins(orange) to regulate passage of molecules between cells.
enveloped virus membrane fusion for cell entry
fusion between viral and cellular membranes must occur to deliver the viral nucleic acid into the cell
Membrane fusion takes place during many normal cellular processes, such as cell division, myoblast fusion, and exocytosis, and must be regulated in order to maintain the integrity of the cell and its intracellular compartments.
Consequently, membrane fusion proceeds by specialized mechanisms mediated by proteins and requires energy. Some of the best-characterized fusion machines are viral envelope glycoproteins.
Despite many differences in details, the fusion mechanism is remarkably similar between all fusion proteins from different virus families and relies on conformational changes in the viral protein or subunit that mediates fusion.
influenza viral entry -class 1 entry
The globular heads of HA trimers mediate binding of the virus to sialic acid-containing cell receptors.
The virus-receptor complex is endocytosed and the import of H+ ions into the endosome acidifies the interior.
Upon acidification, HA undergoes conformational rearrangements that produce a fusogenic protein.
Insertion of the fusion peptide into the target membrane results in the adoption of an extended intermediate structure.
This structure bridges the two membranes but does not bring them close enough for fusion to occur
structure of class 1 viral fusion proteins
hairpin structure forms after the fusion trigger of membranes - folding into hairpin decreases the distance between viral and cell membranes and permits membrane fusion
class 2 fusion proteins
- coiled coils
-The proteins share a common fold with a centralβ-sandwich domain I flanked by domains II and III that tile the surface of the virus particles as dimers (A).
-(A) Ninety dimers of dengue virus envelope glycoprotein E tile the surface of the virus particle at neutral pH.
(B) At low pH, the dimers are disrupted; the proteins extend so that the fusion loop inserts into the target membrane and reorganizes into trimers.
The glycoprotein then undergoes further conformational rearrangements, folding domain II against domain I, which brings the viral and cell membranes together, allowing fusion.
class 3 fusion proteins
This class is exemplified by the G protein of the rhabdovirus vesicular stomatitis virus and the gB proteins of herpesviruses
The structural organization, shared by class III fusion proteins, is rather complex, with three domains (I to III) nested around a β-sandwich core and along C-terminal extension.
The transition to the fusion active state consists of rotation of domains I and II and refolding of domain III to form a hairpin, similar to that described for class I fusion proteins, and projects two fusion loops toward the target membrane. Class III Fusion Proteins
poliovirus entry into the cell
the native particle binds to the CD155 cell receptor and undergoes a transition resulting in altered A particles
after endocytosis the viral RNA leaves capsid - a long umbilical connection is formed between the particles and the endosomal membrane allowing it to escape
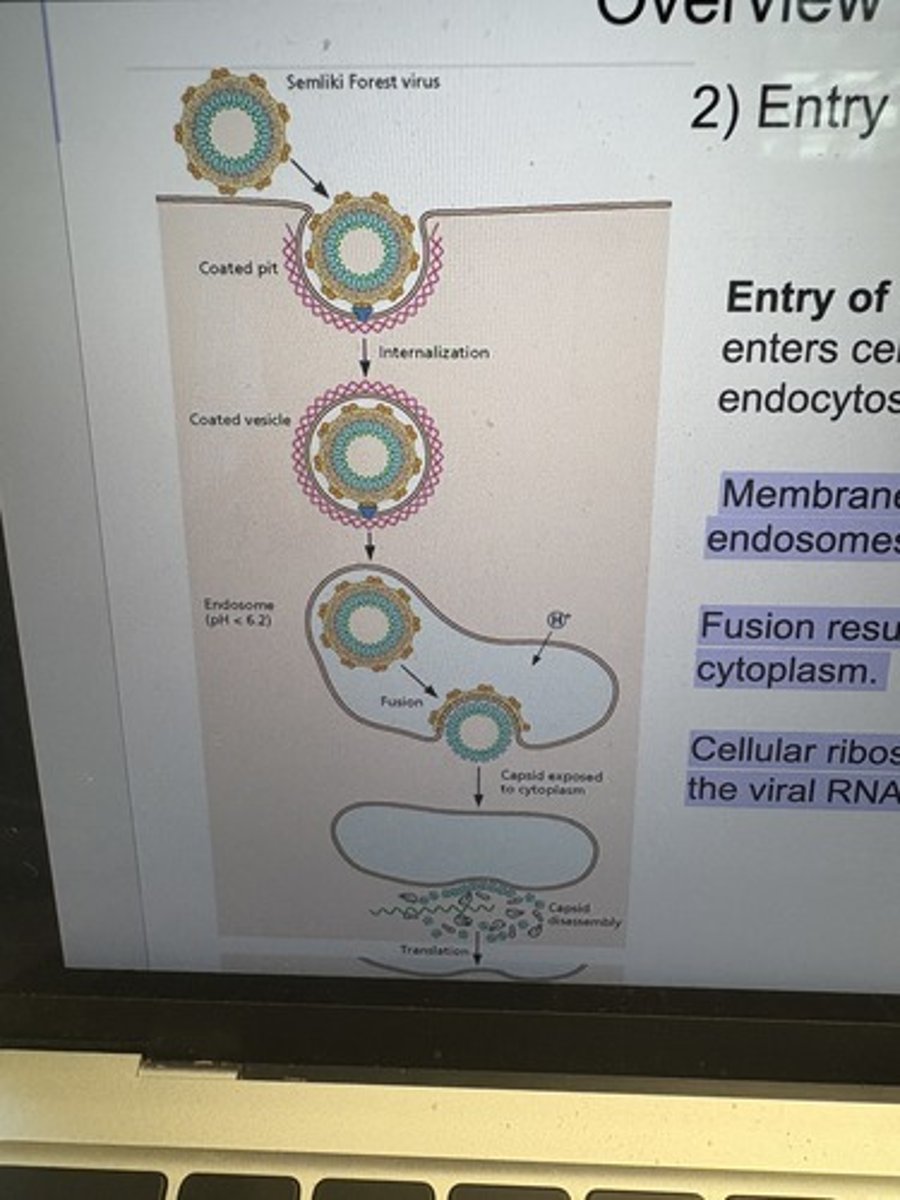
Entry of Semliki Forest virus into cells.
Semliki Forest virus enters cells by clathrin-dependent VLDLR-mediated endocytosis.
Membrane fusion is catalyzed by the acidification of late endosomes.
Fusion results in the exposure of nucleocapsid to the cytoplasm.
Cellular ribosomes bind and disassemble the capsid, rendering the viral RNA accessible to translation.