Cytoskeleton , cell adhesion and cellular motility
1/29
Earn XP
Description and Tags
To be able to describe the three major structural components of the cytoskeleton, their assembly and disassembly and their associated proteins. To understand the concept of assembly by polymerisation of globular subunits. To understand the importance of the cytoskeleton in various aspects of cell morphology and structure, intracellular motility and cell migration.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
What are the functions of the cytoskeleton?

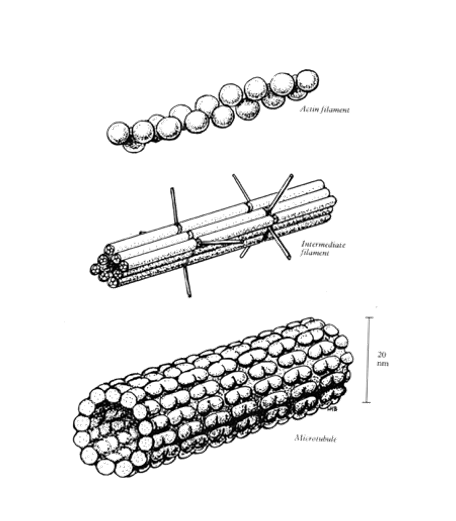
What are the 3 different classes of protein polymers in the cytoskeleton?
Actin (microfilaments) 7nm diameter
Intermediate filaments 10nm diameter
microtubules 20nm in diameter
Associated proteins may bind t the filaments, The wide variety of associate proteins allows these 3 basic structures to perform diverse functions
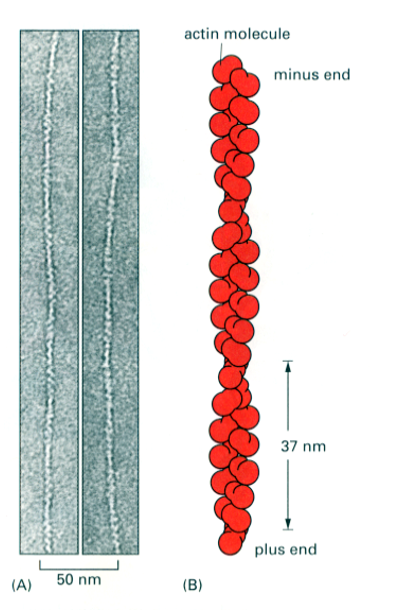
What is the structure of Actin?
Actin filaments (long polymers) are called F actin (filamentous actin or microfilaments)
Individual actin proteins are called G actin (globular actin)
The actin filament is a polarised double helix
There are 13 actin subunits (G actin) for every complete turn of the helix) 37nm pitch
The asymmetric shape of the actin monomer gives polarity to the actin filament (+ and - ends)
The combined weight of both F and G actin makes up 5% of the total protein weight in a cell
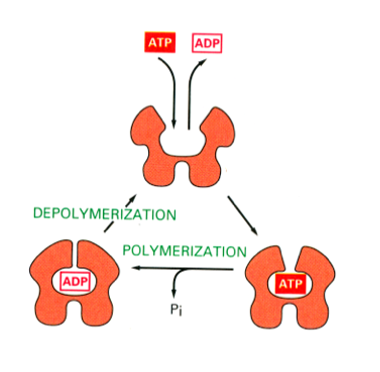
Growth and assembly of the actin filament
Requires ATP to be bound to G actin (monomer) , can be added onto either end of the actin polymer but faster as the + end
Filaments are very dynamic, constantly extending and contracting. Once incorporated into the filament, the ATP is hydrolysed to ADP
Actin filaments are very dynamic
During processes such as cell migration and wound healing
Major functions of actin
Mechanical support (eg stereocilia)
Changing or maintaining cell types (eg. maintaining the biconcave shape of RBCs)
For cell motility (all moving cells and growing nerve cells use actin to move, muscles use actin to contract)
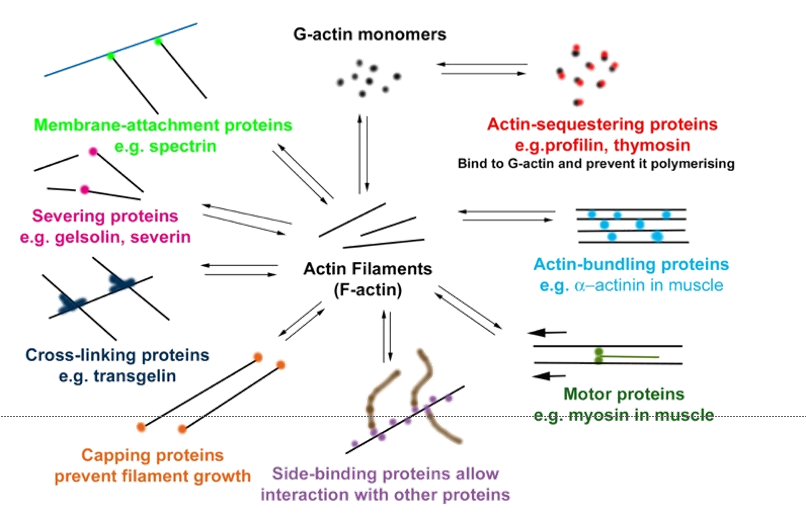
A wide variety of actin-binding proteins allow the actin filaments to interact with many different cytoplasmic proteins - actin’s diverse and essential roles.
Structure of intermediate filaments
Rope like polymers of intermediate filament proteins. 10nm in diameter
Heterogenous protein family (variety). Detailed molecular composition may vary be cell type eg keratin (epethelia) GFAP (glail)
Cytoplasmic intermediate filaments form a network. Typically most dense around the nucleus
Functions are mainly mechanical. They also anchor cells at some cell junctions: desmosomes & hemidesmosomes
A particular type of IF, lamins, inside the nucleus, support nuclear structure & protect chromatin.
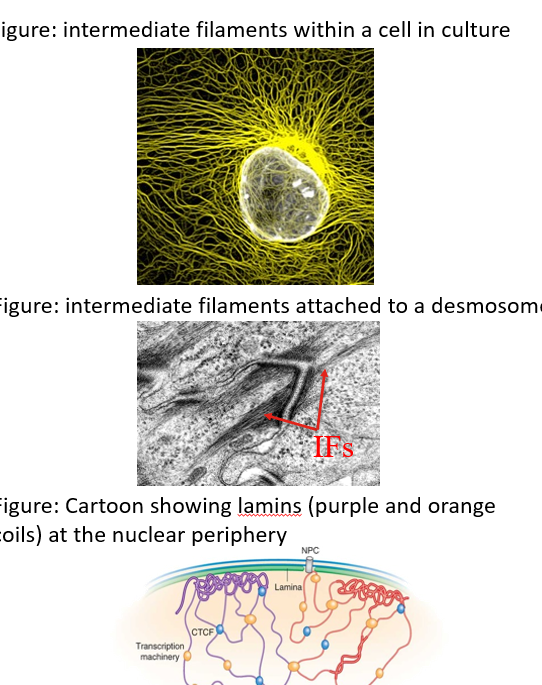
Formation of intermediate filaments

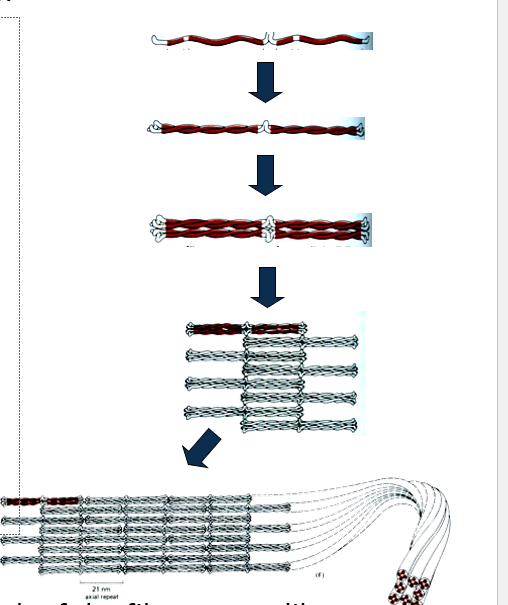
How is subunit exchange different in intermediate filaments compared to microfilaments and microtubules?
Subunit exchange is slow but occurs throughout the length of the filament, unlike actin and tubulin, which exchange only at filament ends
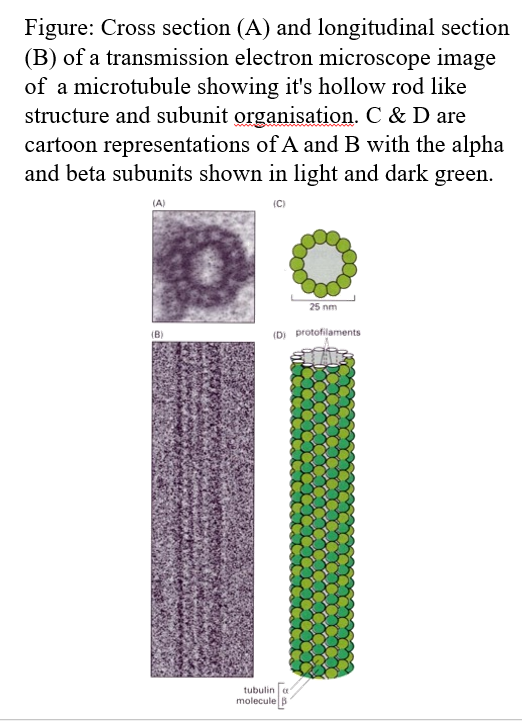
Microtubules structure
Long, relatively stiff hollow tubes
20-25nm in diameter
Can be rapidly dissembled and reassembled
There is a + and - end
The polymer rod is built from monomers of tubulin.
There are 13 polymer rods seen in cross-section of a microtubule.
Tubulin “monomer” consists of one molecule of a-tubulin and one of b-tubulin (each of these is a different protein encoded by a different tubulin gene)
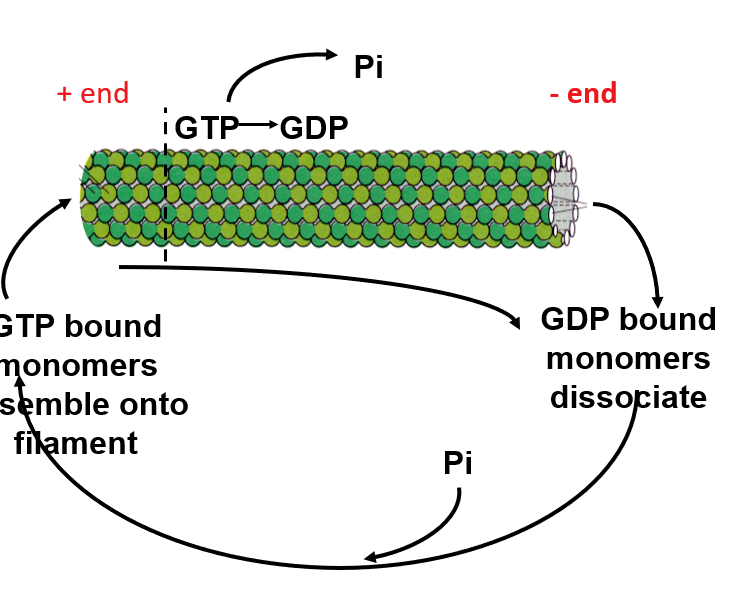
Assembly and disassembly of a microtubule
The alpha/beta monomer is assymetric: alpha one side and beta the other. (polarised)
GTP-bound alpha/beta monomers are added to the plus end of the microtubule with GTP being converted to GDP
GDP-bound monomers detach at both the the minus and plus ends end of the microtubule
This happens more quickly at the +end
Actin involved in cell shape and polarity
Actin filament bundles provide support e.g for microvilli in the gut and for stereocilia to detect vibration of sound waves in the cochlea They are found beneath the plasma membrane, a region called the cortex of the cytoplasm. These maintain cell shape in red blood cells (erythrocytes)
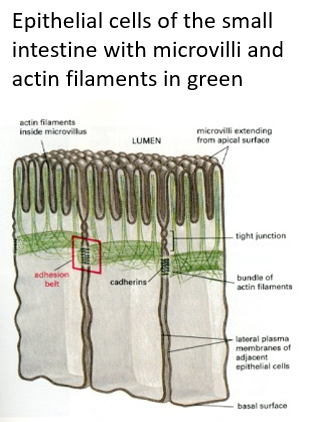
intermediate filaments involved in cell shape and polarity
Stabilise long cell processes such as axons of nerve cells
Microtubules involved in cell shape and polarity
Also stabilise mature axons but are also involved in their growth as the brain develops Stabilise the irregular shapes of platelets, a property related to their role in blood clotting
Microfilaments involved in anchoring and organising organelles
Actin tethers vesicles full of neurotransmitter close to the presynaptic membrane of synapses in the nervous system
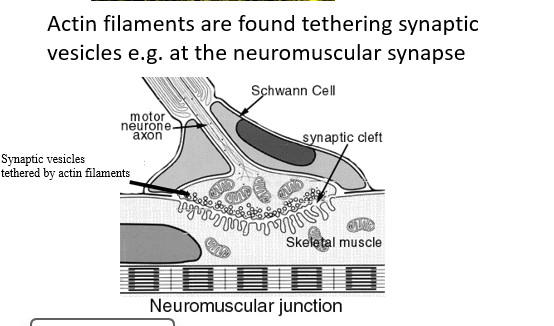
Intermediate fibres involved in anchoring and organising organelles
Intermediate filaments form a meshwork around the nucleus to anchor it in position
Microtubules involved in anchoring and organising organelles
Microtubules organise the endoplasmic reticulum
How is the cytoskeleton involved in anchoring CELLS?
The cytoskeleton is essential to bind cells to their neighbours at intercellular junctions (adhesion belts and desmosomes). And to underlying extracellular matrix (hemidesmosomes)
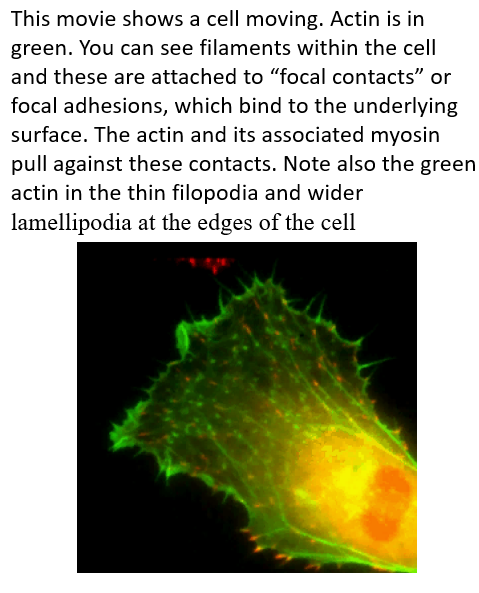
Actin based cell movements- four events
Cell pushes out protrusions at the front (leading edge). Actin filament polymerisation provides the force for membrane protrusion.
The protrusions adhere to the surface on which the cell is moving through focal adhesions (usually extracellular matrix) F-actin connects to the focal adhesions to provide a contractile force for the cell.
The rest of the cell pulls against the anchorage points to drag itself forward.
Actin depolymerises at the rear.
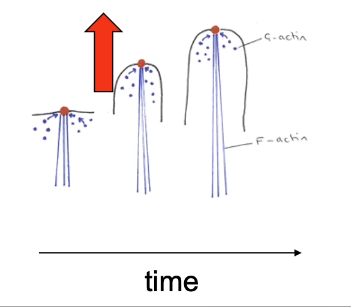
What is filopodia and what is its role in cell motility ?
Filopodia are finger like protrusions that sample the environment and extend and withdraw, generated by the rapid growth of actin filaments at the plasma membrane + end is towards end of the cell
How do you generate a contractile force (actin base motility)
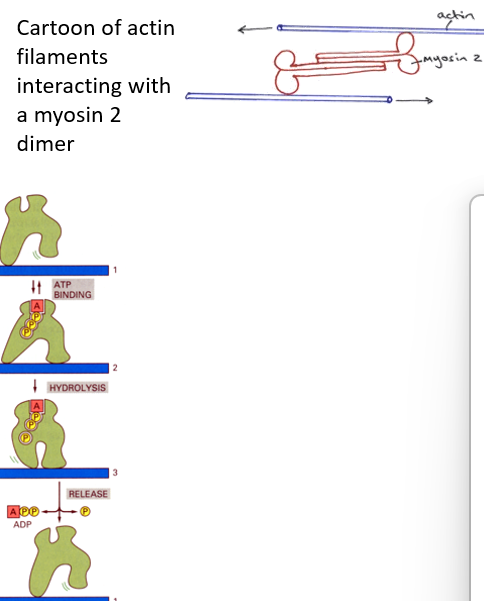
The actin filaments are pulled upon by myosin to drag the cell forward
The myosin used is non-muscle myosin-2.
Myosin is one a class of proteins called MOTOR PROTEINS
The “head region” of the myosin interacts with actin and binds ATP. Energy release from ATP hydrolysis forces the myosin tail to move, generating forces.
ADP is released from the myosin head and replaced by ATP. At this stage the head can detach from the actin filament.
The head now binds further down the filament.
Cell motility - microtubule based movements
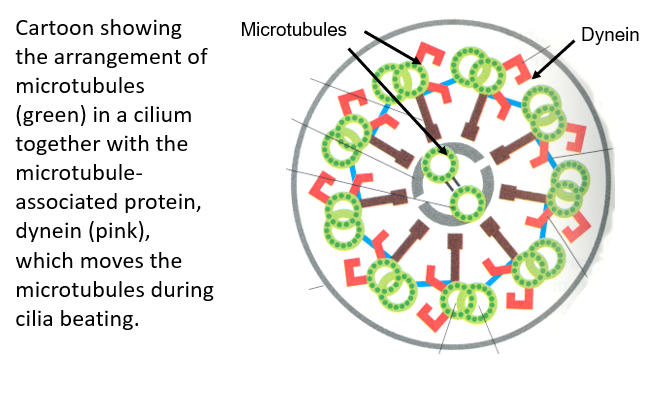
Microtubules produce the movement of cilia and flagellae e.g. the cilia found on respiratory epithelial cells
Microtubule rods are organised into a “9 + 2” arrangement within respiratory epithelia
Dynein is responsible for the ,movement of the microtubule rods
Microtubules slide along one another, causing the cilium to bend
Movement of intracellular contents and organelles: microtubules
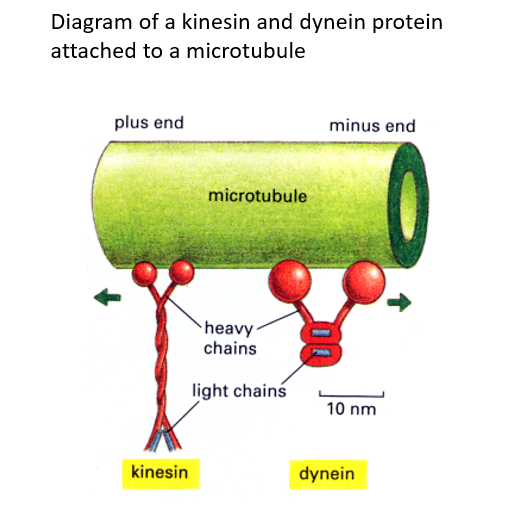
Movement is initiated by the microtubule associated protein, Dynein; a minus end-directed motor protein.
Movement of organelles e.g. synaptic vesicles along axons to synapses
Two motor proteins kinesin and dynein move cargo like vesicles along microtubules.
Kinesin moves towards microtubule + ends (cell periphery)
Dynein moves towards – ends (near nucleus).
Vesicles move 10 cm per day so can take more than a week to move down long axons
Separation of chromosomes during cell division by microtubules
Cytoskeleton in disease and therapy
Chemotherapeutic agents
The chemical compounds colchicine, vinblastine and taxol are all anti-cancer therapeutics.
Colchicine and vinblastine destablise microtubules
Taxol stablises microtubules.
All three inhibit the function of the mitotic spindle (previous slide)
…… and thus have been used to inhibit cell division/proliferation of cancer cells
Defective actin-associated proteins
Mutations in Dystrophin gene, an actin-associated protein cause Duchenne and Becker Muscular Dystrophy
Myosin VII mutations cause Usher’s Syndrome – hereditary deafness and blindness; the former involving defective stereocilia in the ear

Intermediate filaments - defects
Epidermolysis bullosa symplex (EBS). Mutations in keratin genes results in failure to form proper keratin filaments in epidermis. Skin is highly sensitive to mechanical injury. Blistering in adults, sloughing of epidermis in newborns
Plectin (a giant protein that can link Ifs, actin and microtubules) mutations also cause EBS with associated muscular dystrophy
Amyotrophic Lateral Sclerosis (ALS, Lou Gehrig’s Disease). Some hereditary forms are caused by mutations in neurofilamin IF genes.
Microtubules defects
Alzheimer’s Disease. Brains of affected individuals display neurofibrillary tangles comprising a microtubule associated protein called Tau. This abnormal form cannot bind microtubules and accumulates in the “tangles”.
Hereditary Spastic Paraplegia. The most common form is caused by mutations in spastin, a microtubule severing protein, causing accumulation of protein in cells
