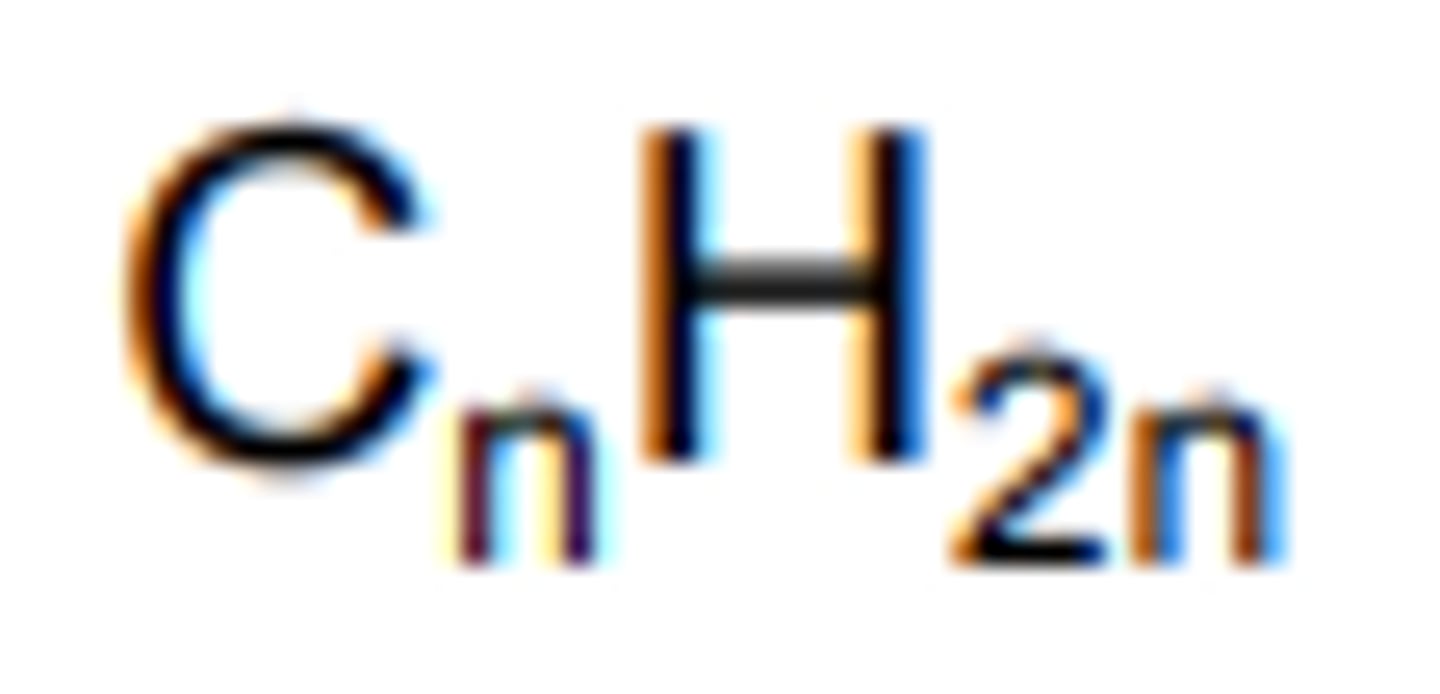CHM255 Exam 1
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
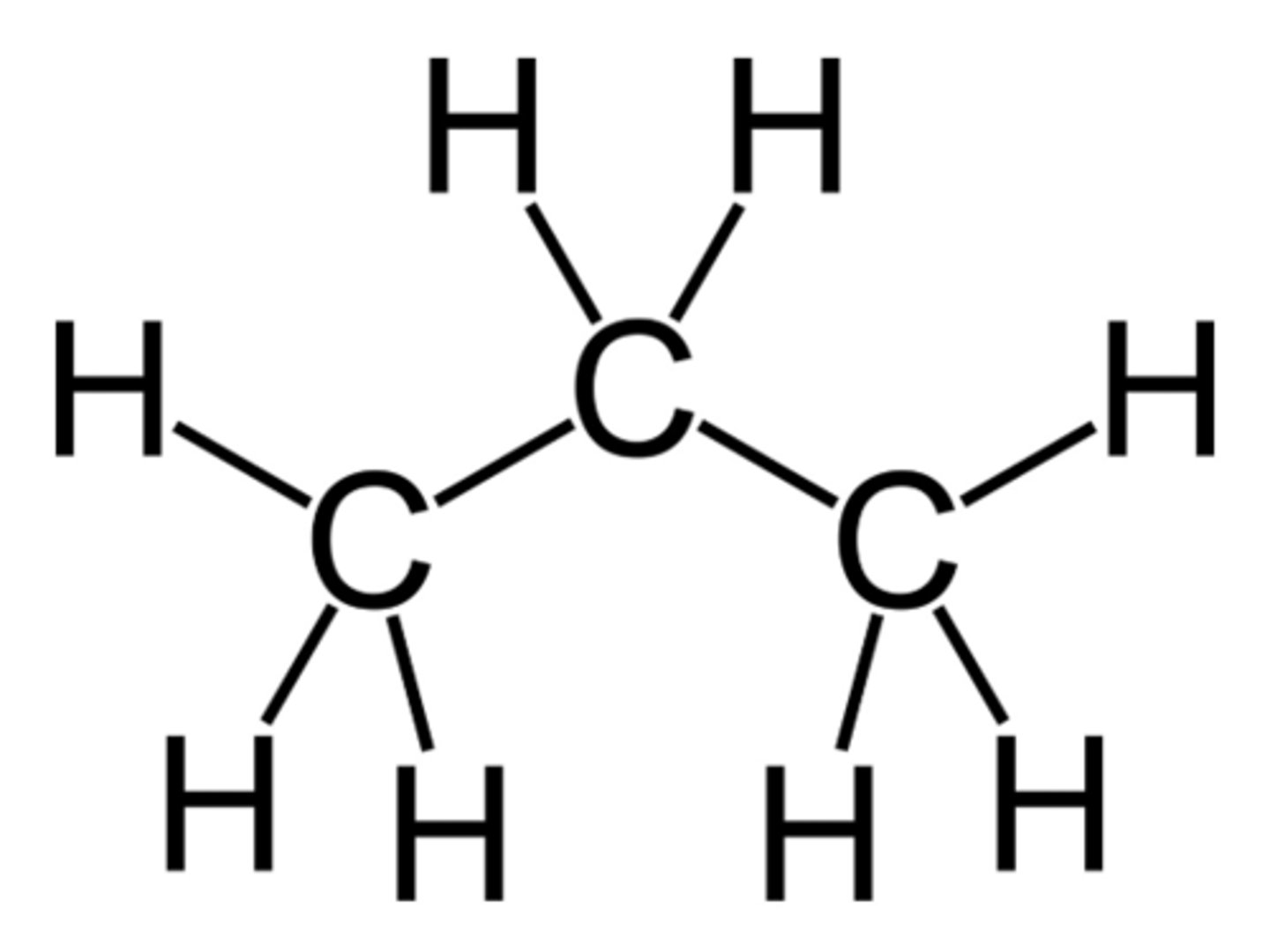
Acyclic Alkanes
Propane
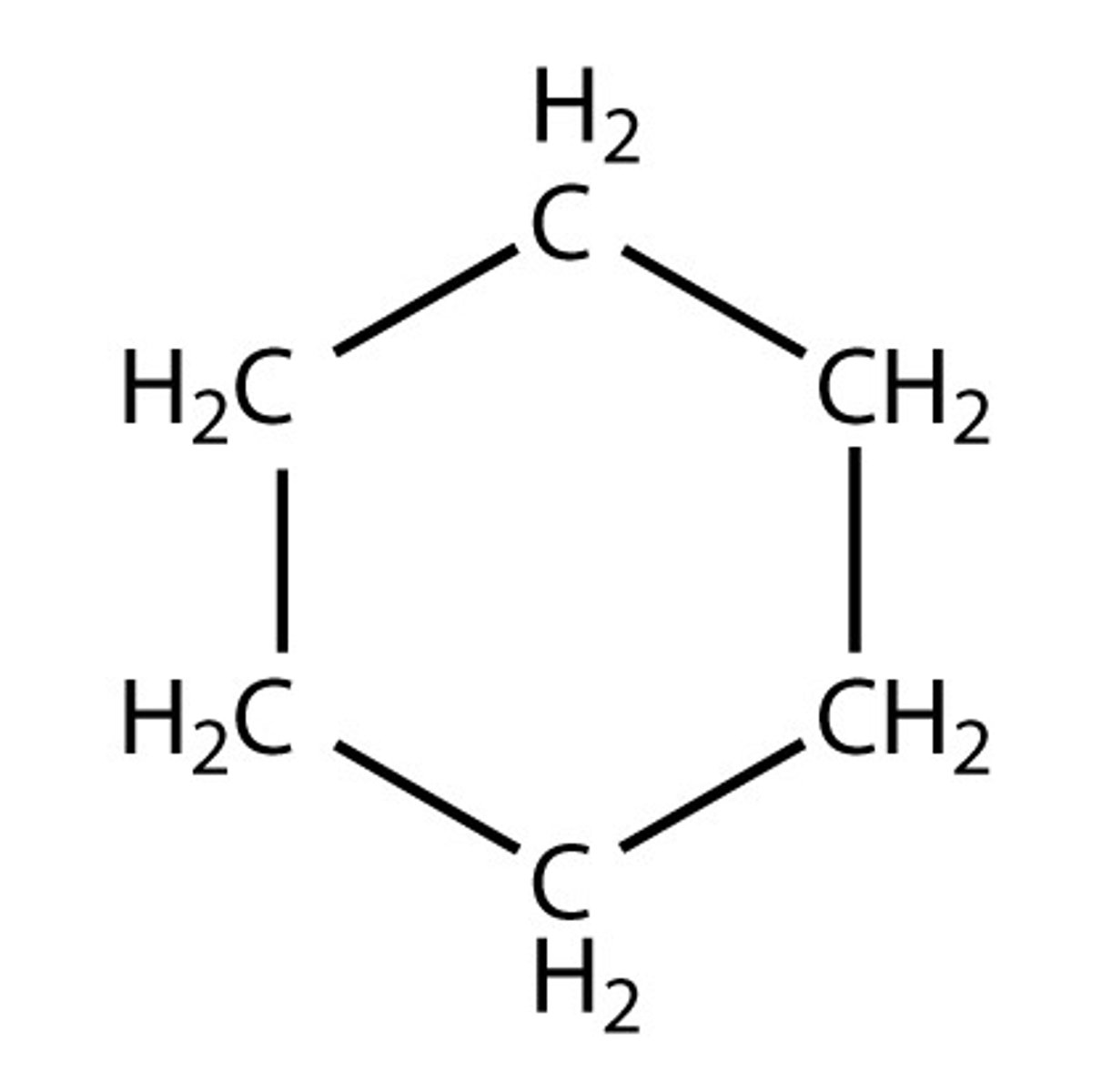
Cyclic Alkanes
Cyclohexane
Alkenes
Hydrocarbons with one or more carbon-carbon double bonds (ethylene)
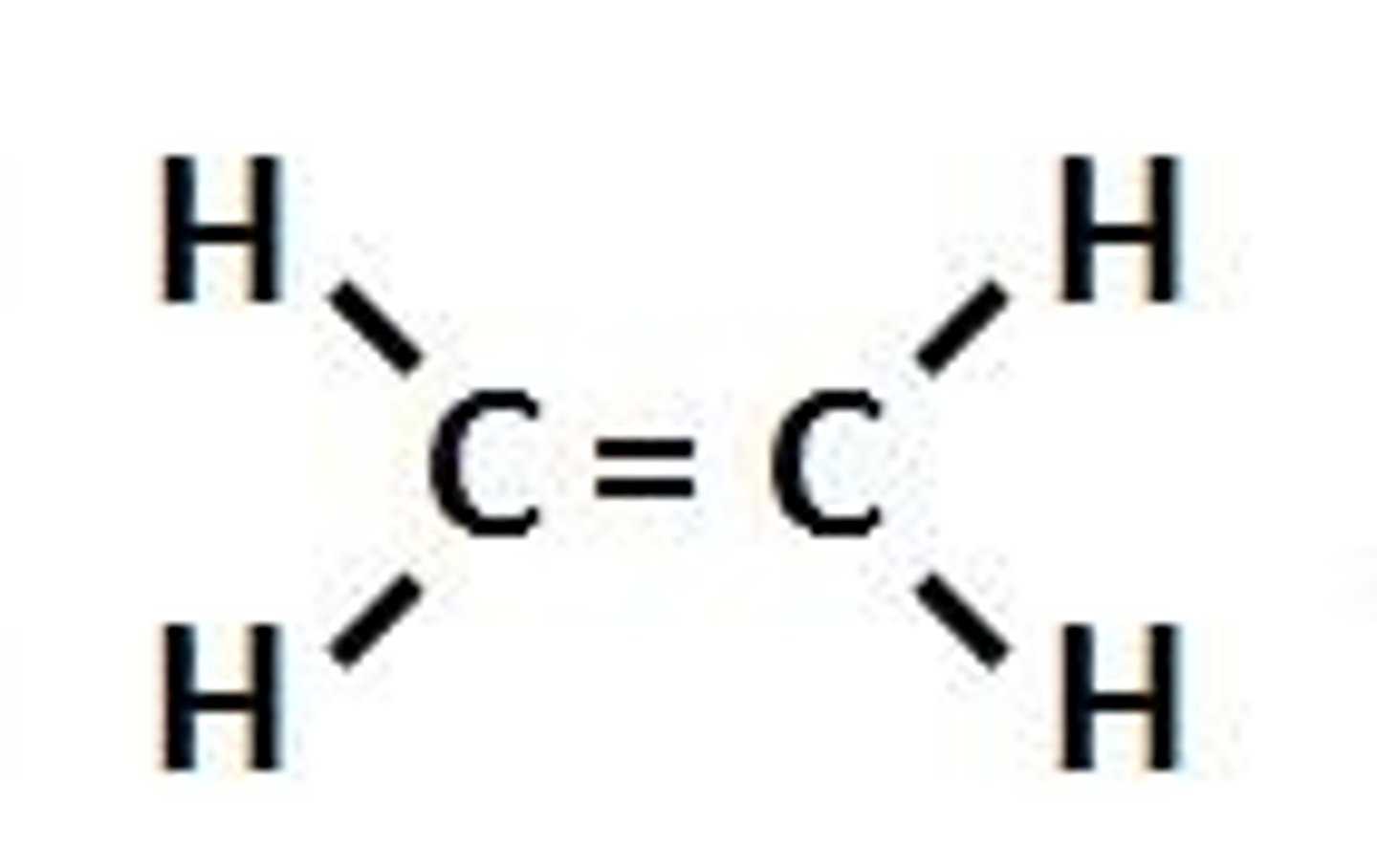
Arenes
Aromatic Rings (Benzene)
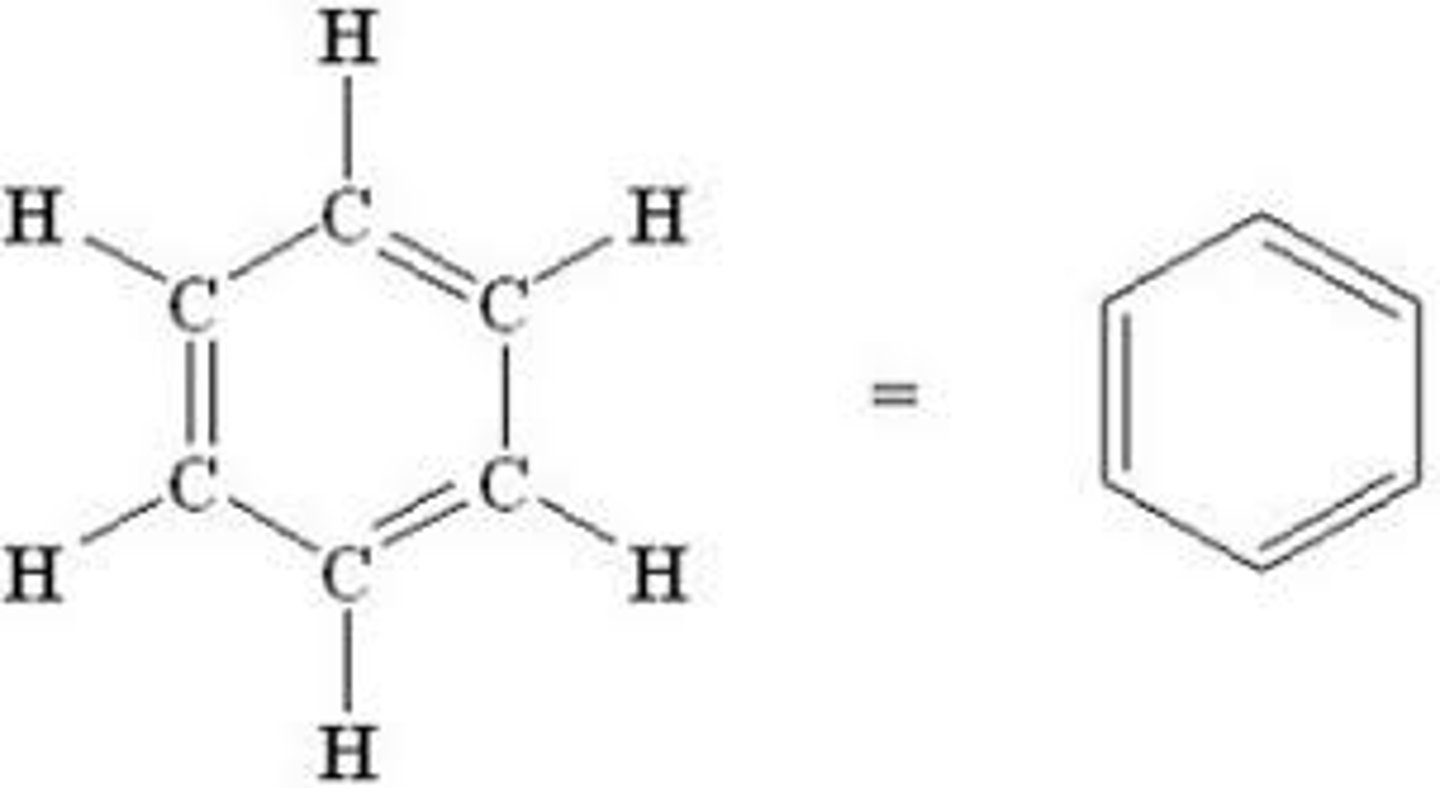
Alkynes
a carbon compound with a carbon-carbon triple bond (ethyne)
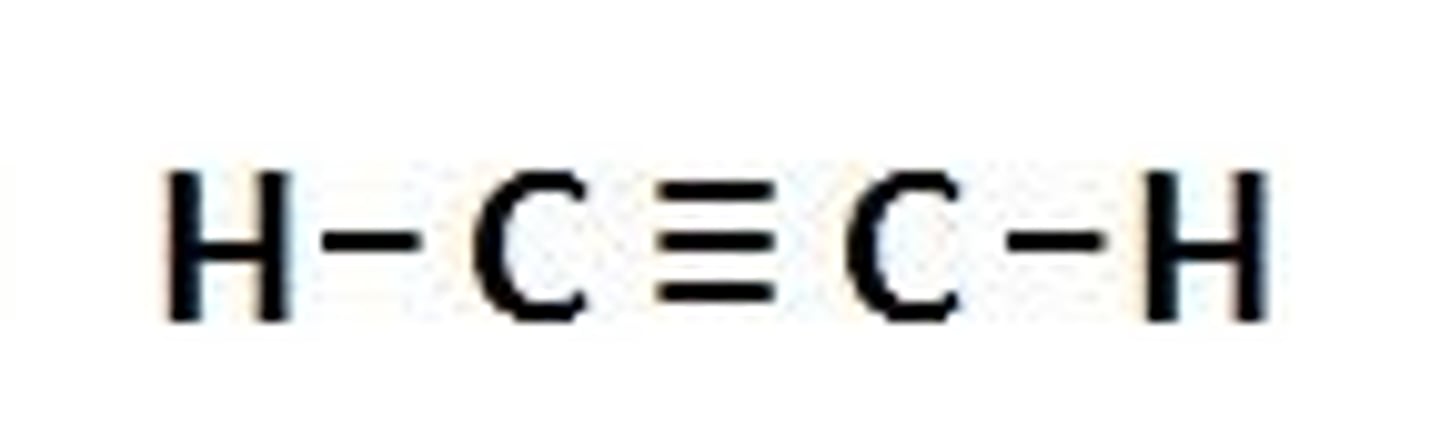
Alcohols
Contain an OH group (ethanol)
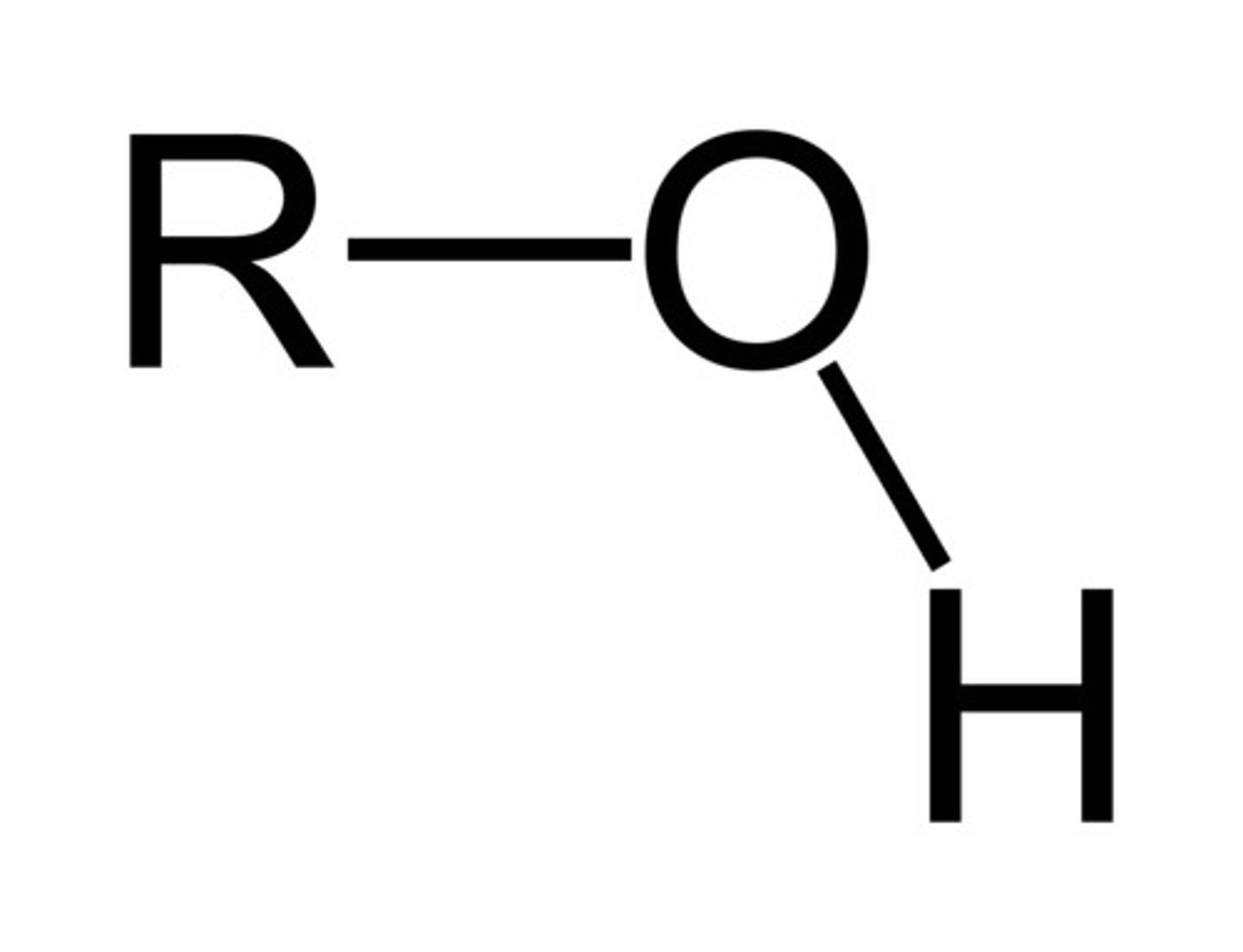
Ethers
C-O-C (diethyl ether)
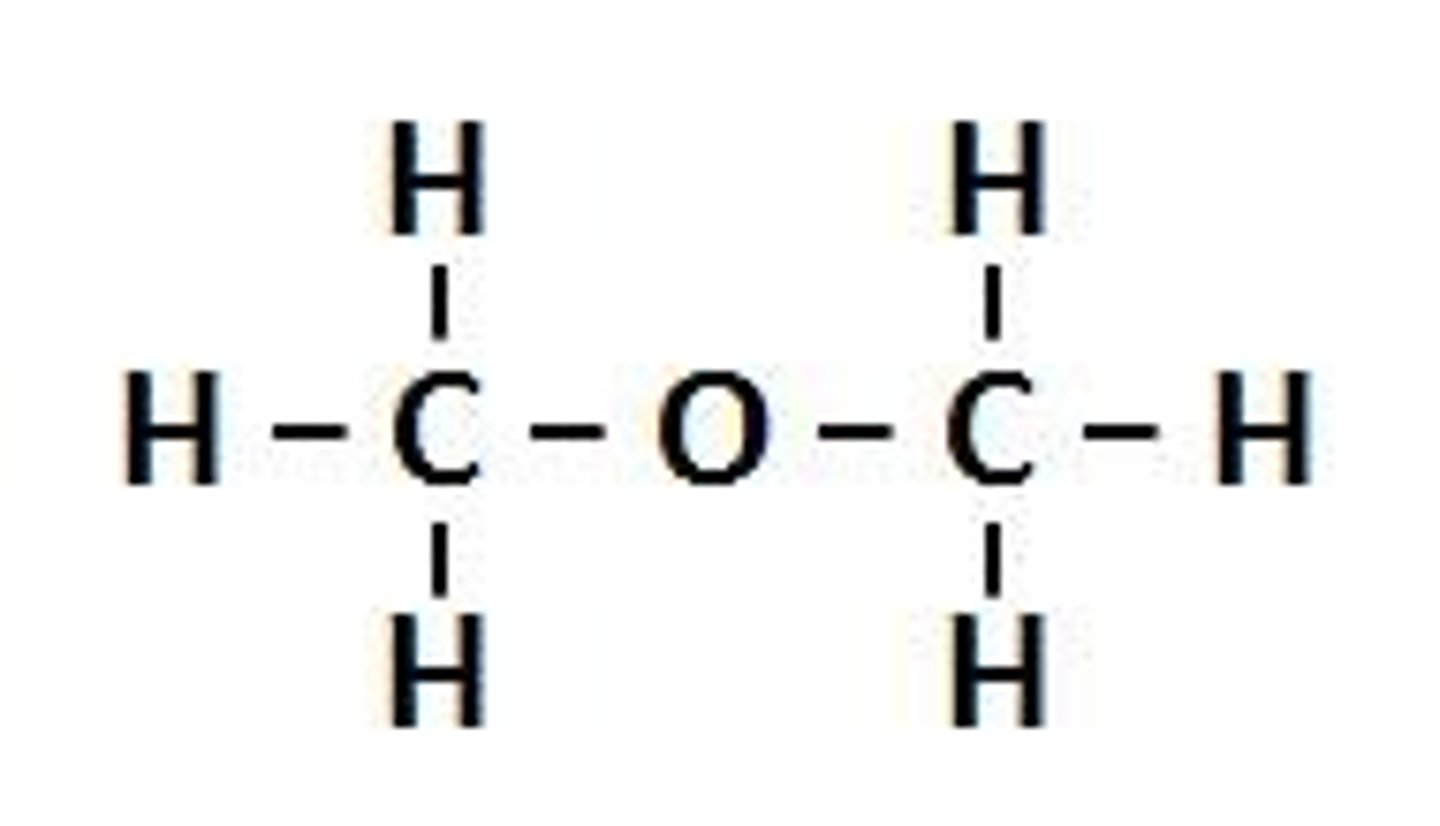
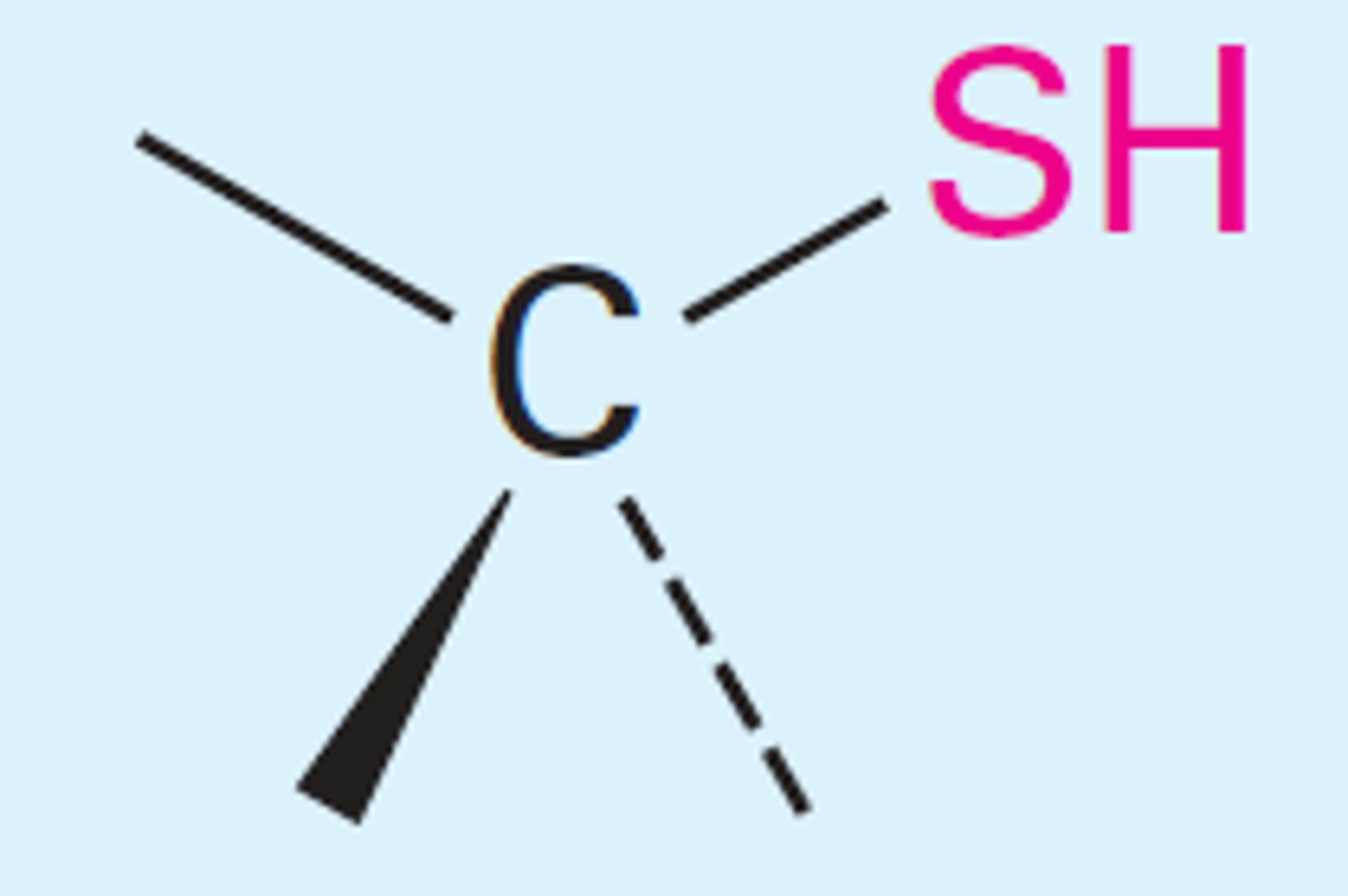
Thiols
-SH
Thioethers
R-S-R

Amines
Contains an NR3 group
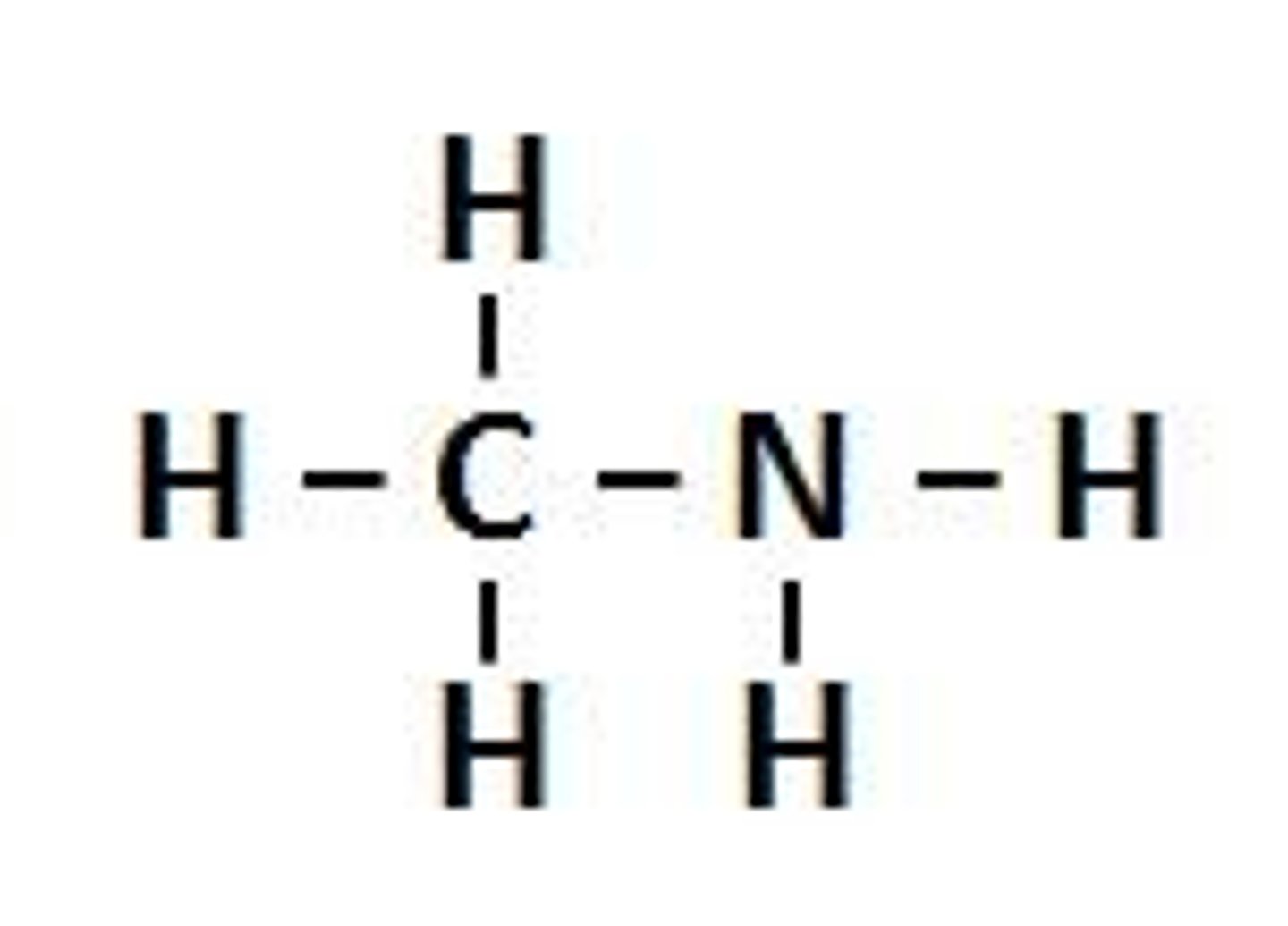
Alkyl Halides
C-X ( X = F, Br, Cl, I)
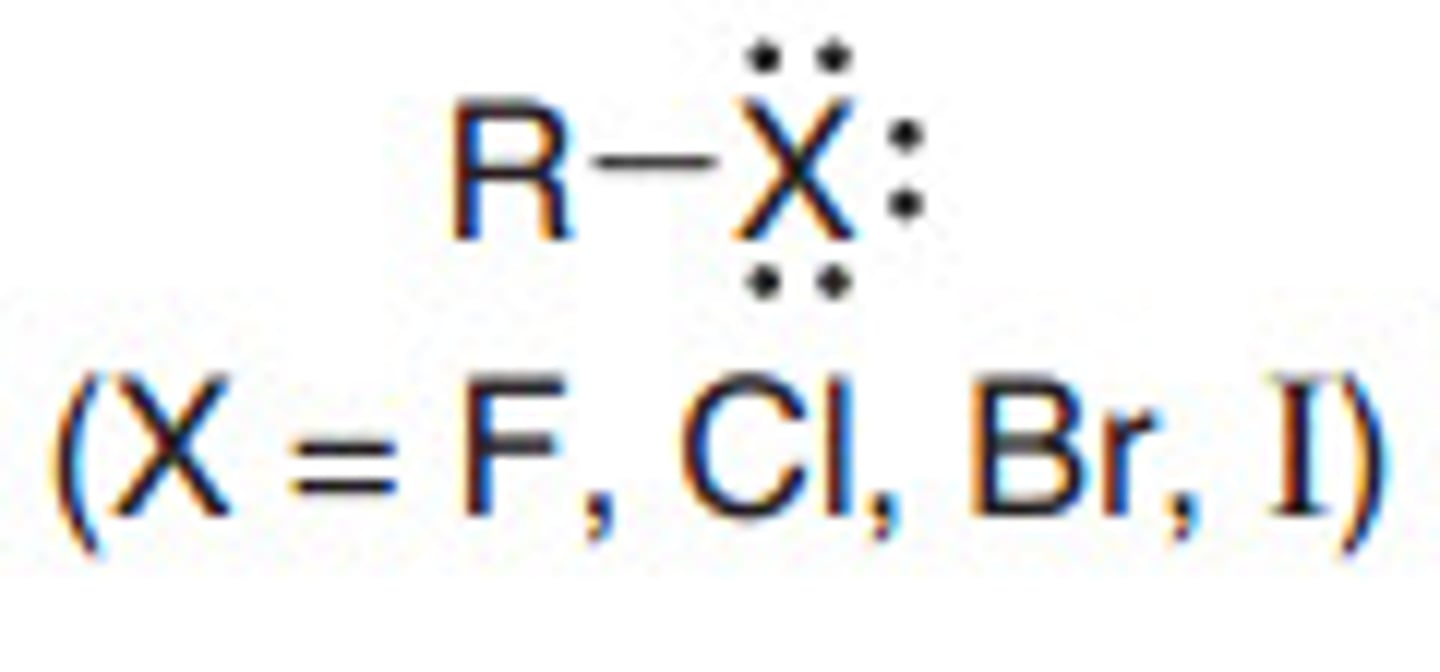
Aldehydes and Ketones
contain the carbonyl group C=O
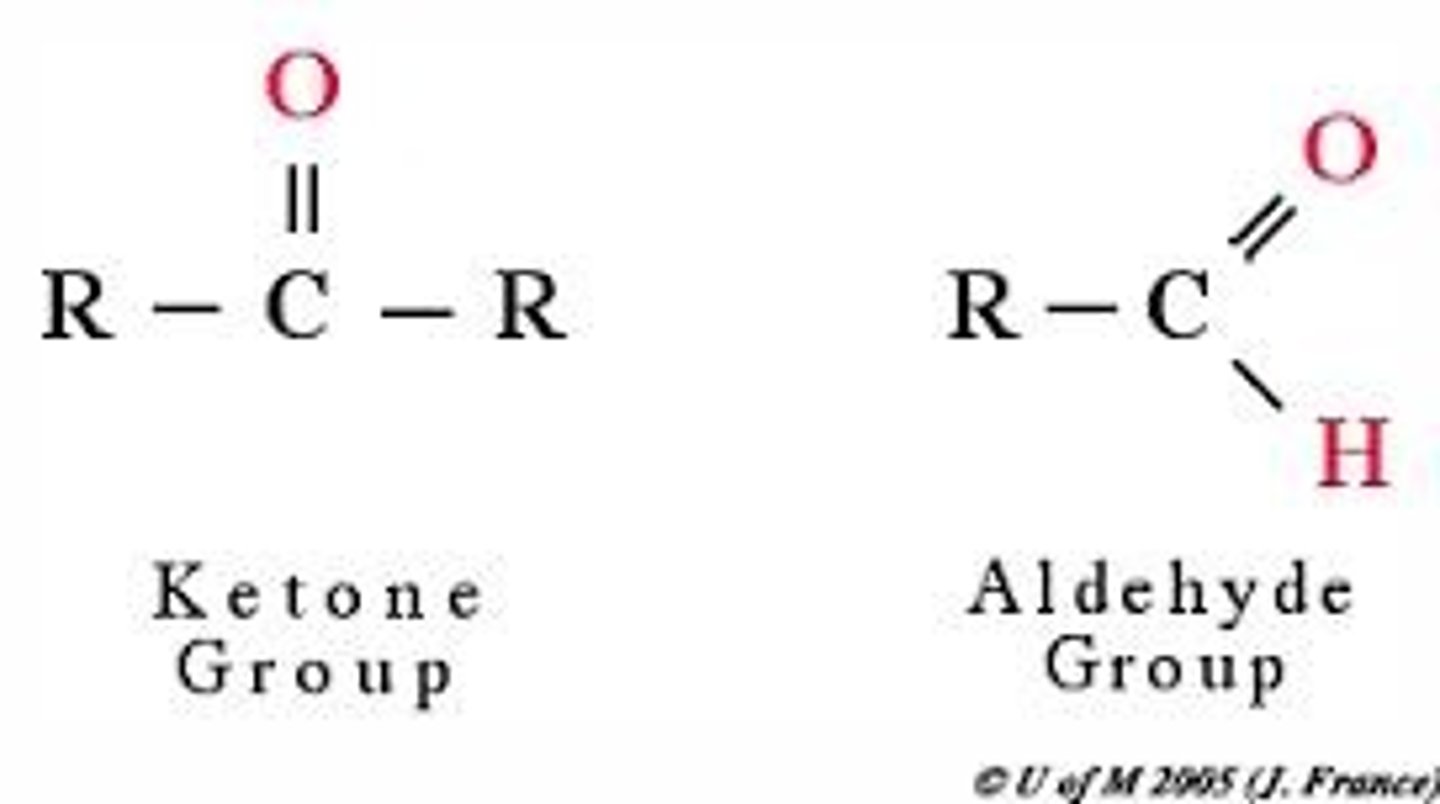
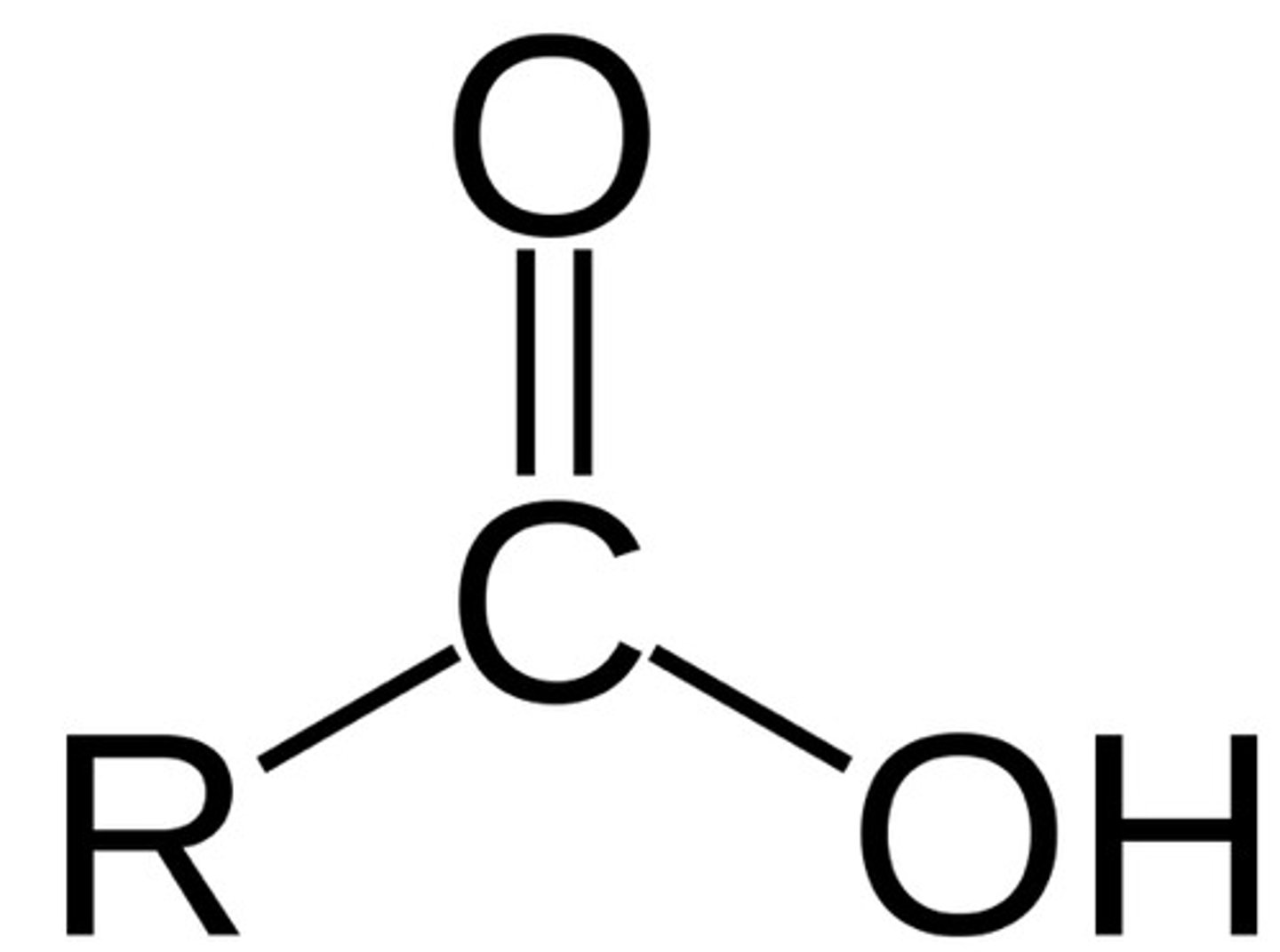
Carboxylic Acid
Contains a carbonyl group (CO) bonded to a hydroxyl group
Esters
Carbonyl Group bonded to an OR group
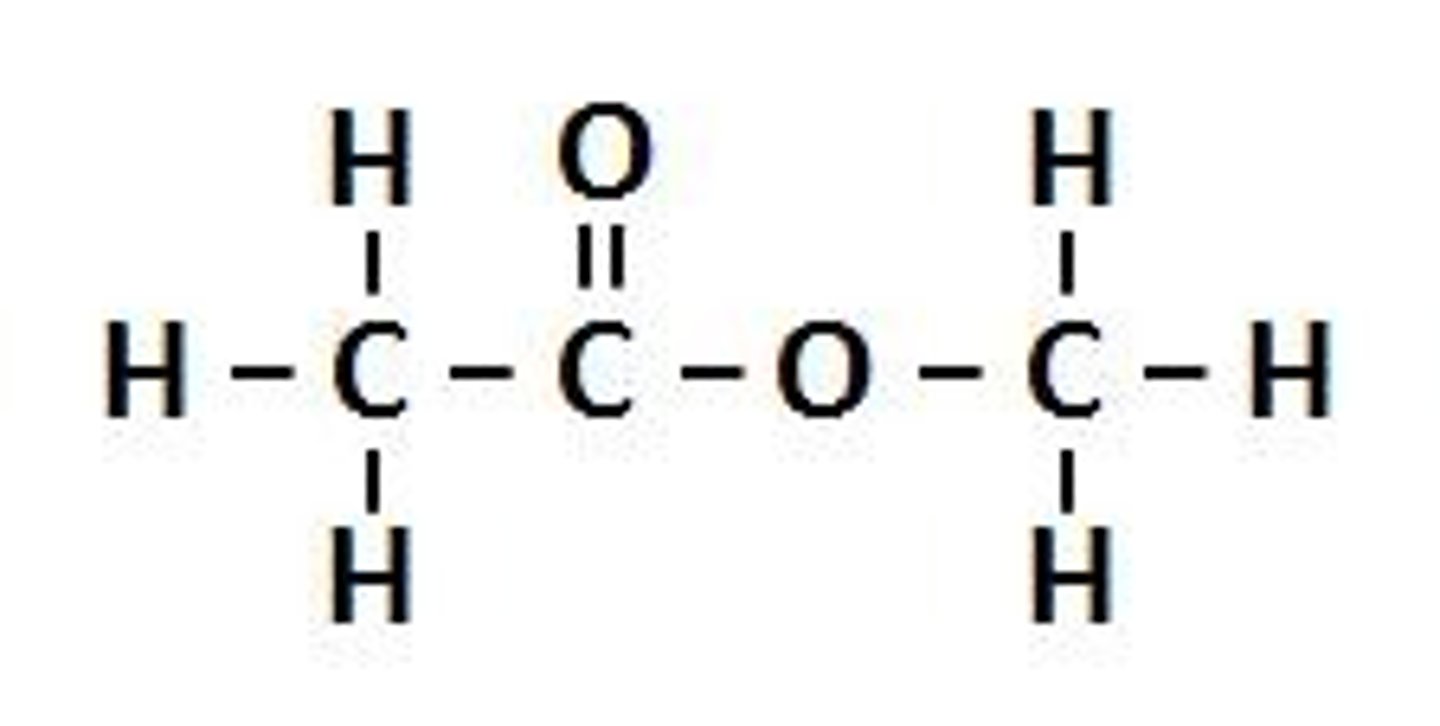
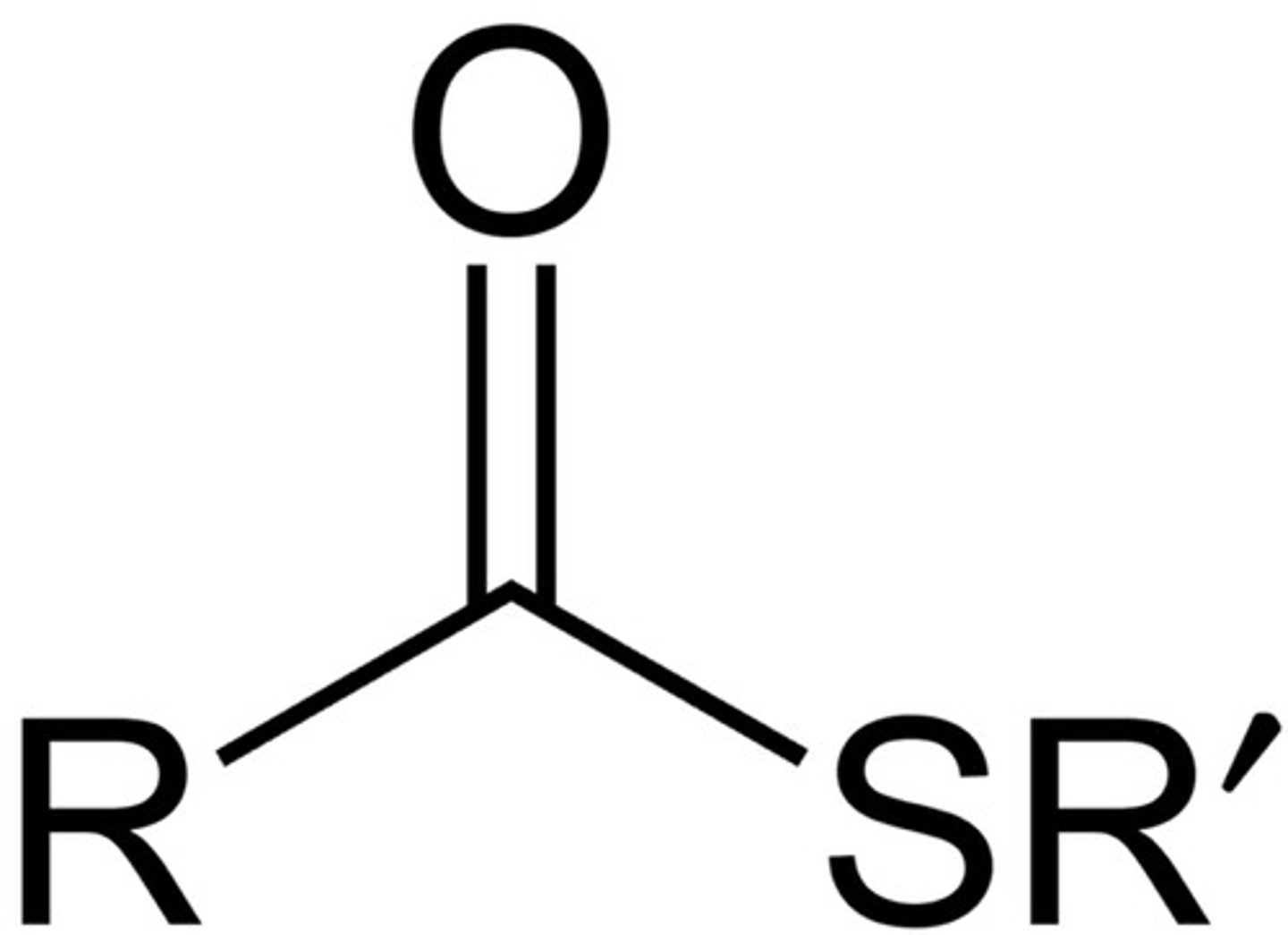
Thioester
Carbonyl group bonded to an SR group
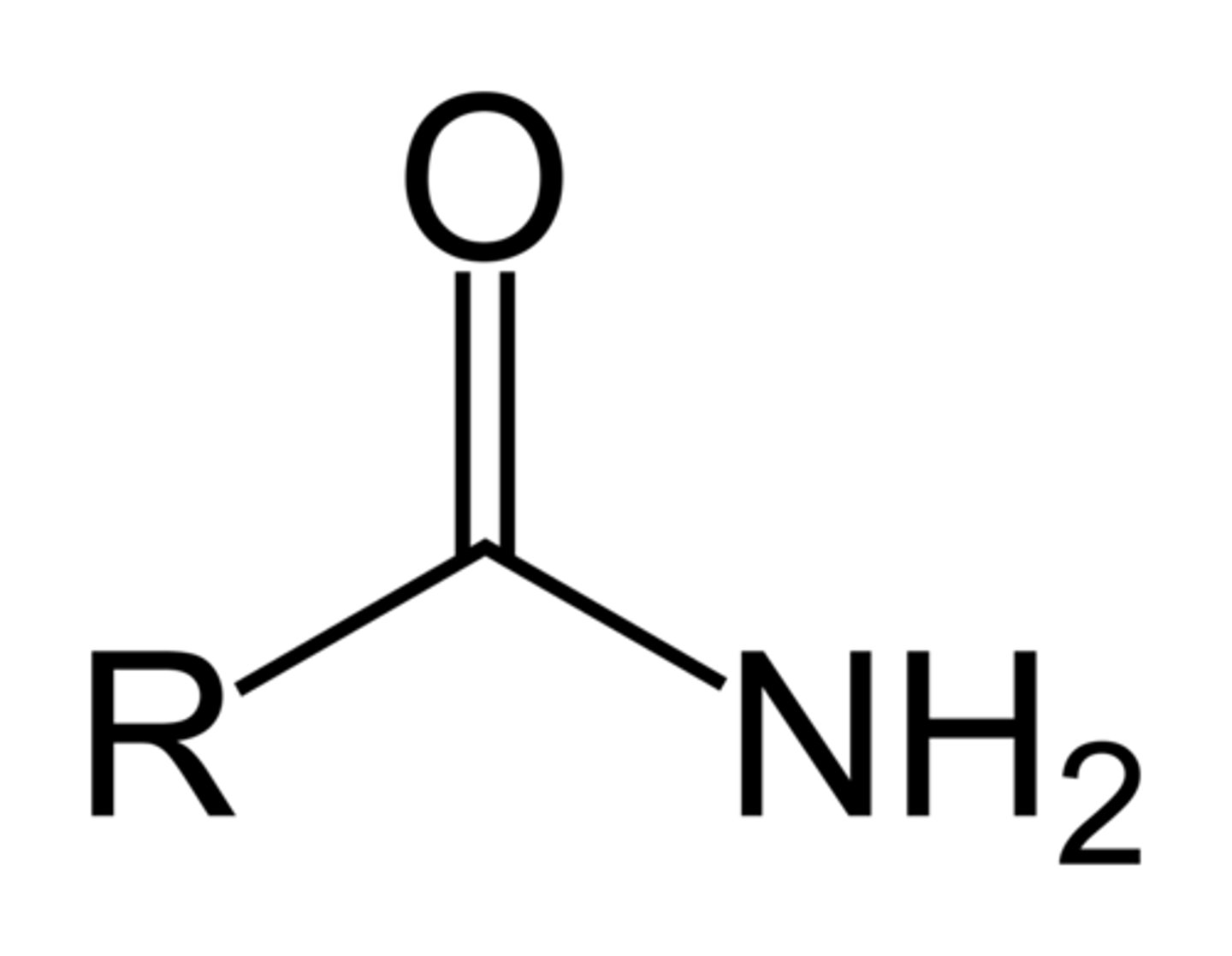
Amide
Formamide
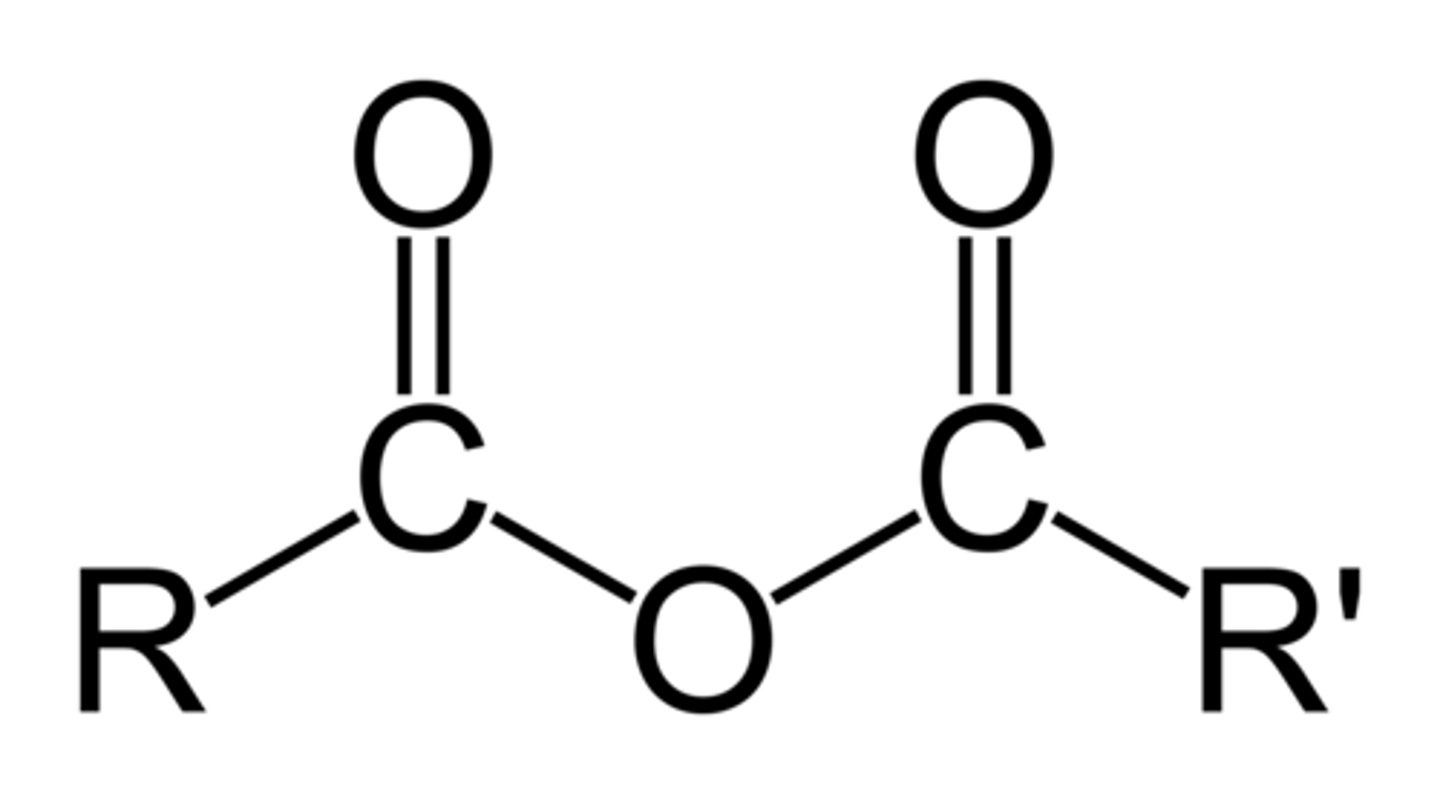
Acid Anhydride
two carbonyls bonded to O
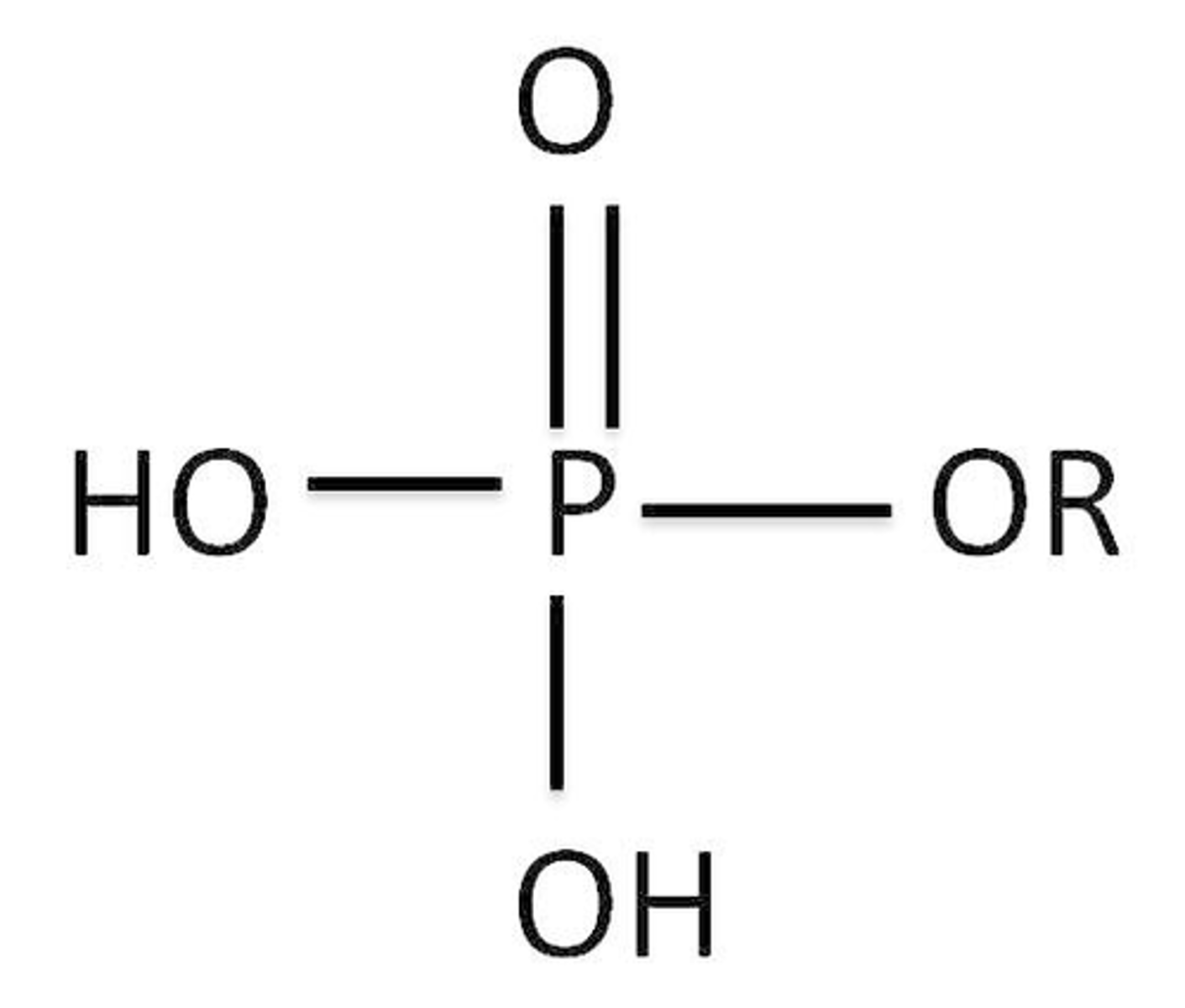
Phosphate Esters
contain a phosphoryl group bonded to a carbon
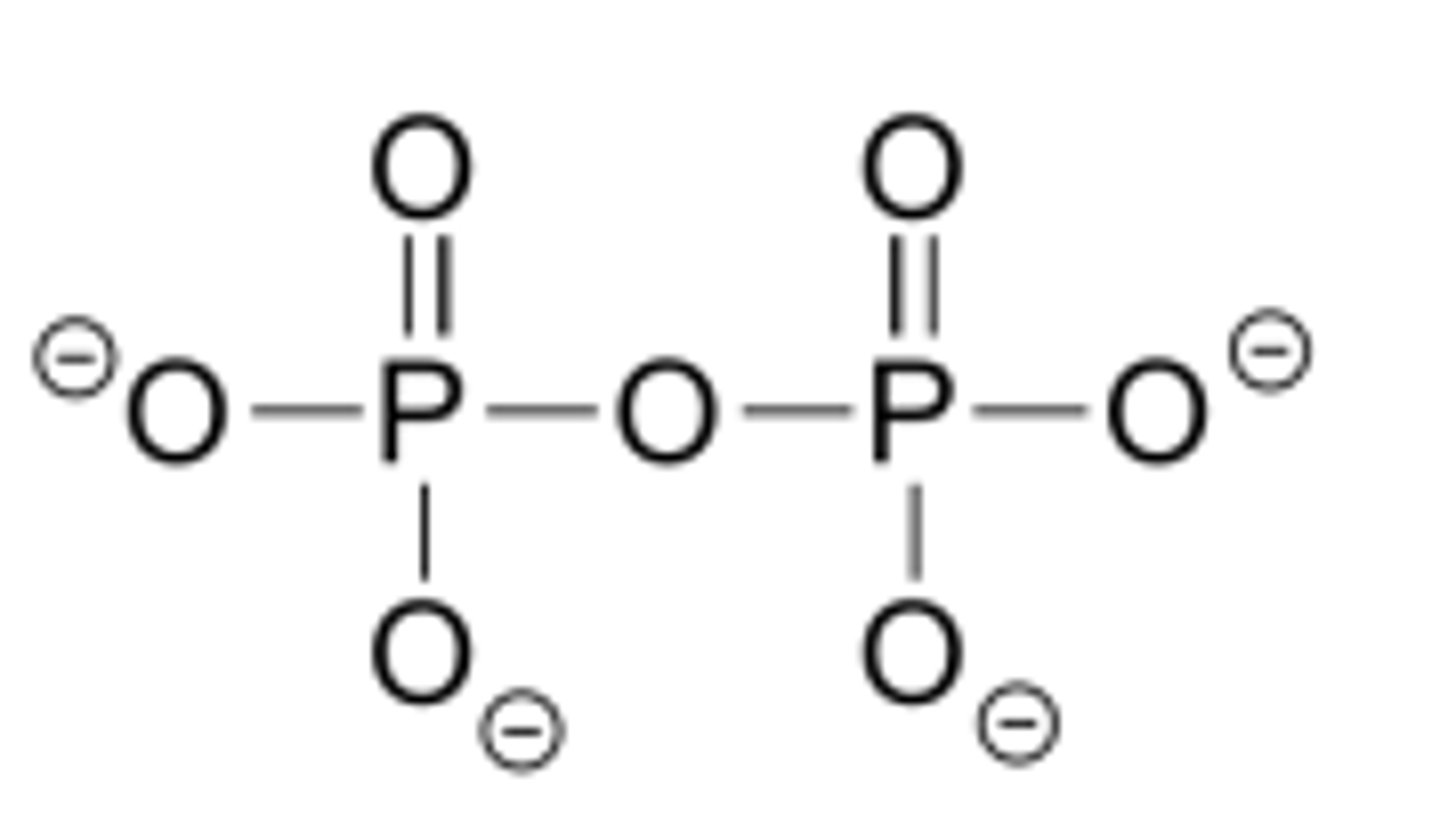
Phosphate Anhydride
contain two phosphoryl groups bonded to an oxygen
Meth-
one
Eth-
2 carbons
Prop-
3 carbons
But-
4 carbons
Pent
5 carbons
Hex-
6 carbons
Hept-
7 carbons
Oct-
8 carbons
Non-
9 carbons
Dec-
10 carbons
Parent
# of carbons in chain
ane-
all single bonds
en-
Double bonds
yn-
triple bonds
-e
alkane
-ol
alcohol
-al
aldehyde
-one
ketone
-oic acid
carboxylic acid
Infix
nature of C-C bond in parent chain
Suffix
class of molecule
1-Locant
In nomenclature, a number used to identify the location of a substituent.
2-Prefix
Where are the substituents
3-Parent
How many Carbons
4-Suffix
What is the primary functional group
R --> R
Forward Reaction
R<---->P
Equilibrium
<--->
Resonance
.---->
Radical (single electron) movement
..---->
Two electron movement
SP3
Tetrahedral 109.5 (4 sigma bond)
SP2
trigonal planar, 120 (3 sigma bond, 1 pi bond)
SP
linear 180 (two pi bonds, two sigma bonds)
Cyclic Alkanes General Formula
CnH2n