Looks like no one added any tags here yet for you.
11.1 Alcohols and Thiols as Brønsted Acids and Bases
Are alcohols and thiols weak acids?
Yes
What are alkoxides?
The conjugate bases of alcohols
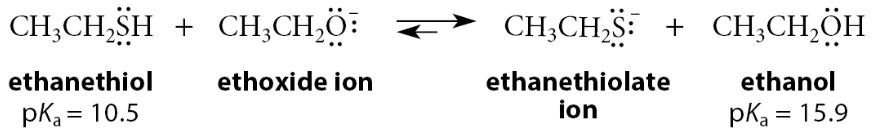
Which one is more acidic: alcohols vs thiols
Thiols are much more acidic than alcohols
What is the element effect?
How can alkoxides be formed?
Alkoxides can be formed irreversibly from alcohols with stronger bases
Are the reactions of sodium with some alcohols slow or fast?
Slow
How can the reactions of sodium with some alcohols be sped up?
The alkoxides of such alcohols can be formed more rapidly with the more reactive potassium metal
What can thiols be converted into?
Converted completely into their conjugate-base mercaptide anions by reaction with one equivalent hydroxide or alkoxide
What is a common method of forming alkali-metal mercaptides?
Dissolve them in ethanol containing one equivalent of sodium ethoxide:
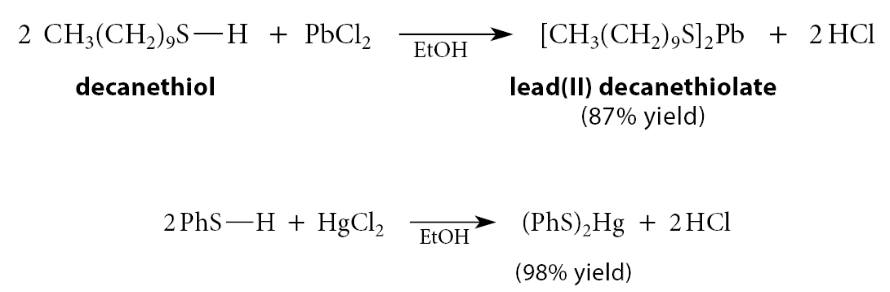
What do thiols form?
Insoluble mercaptides with many heavy-metal ions, such as Hg2+, Cu2+, and Pb2+
What enhances an alcohols acidity?
Alcohols containing electronegative substituent groups
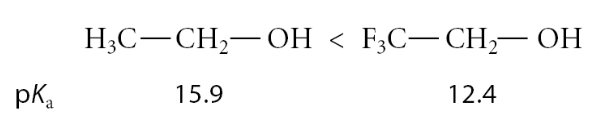
For carboxylic acids, when are is polar effects of electronegative groups are more important?
When the groups are closer to the OH group:
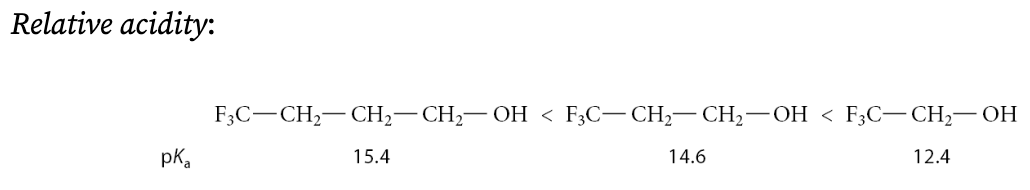
Fluorines separated from the OH group by four or more carbons barely affect acidity
Rank the acidities of the alcohols: tertiary, methyl, secondary, primary
methyl > primary > secondary > tertiary
What does stronger solvation (stronger hydrogen bonding) do?
Stabilizes the conjugate-base alkoxide anion and contributes to increased acidity of the alcohol
What do large branched alkyl groups do?
Interfere with solvation, decrease the stability of the alkoxide, and therefore decrease the acidity of the alcohol
Which is more basic in solution: Tertiary alkoxides or primary alkoxides
Tertiary alkoxides are more basic in solution than primary alkoxides
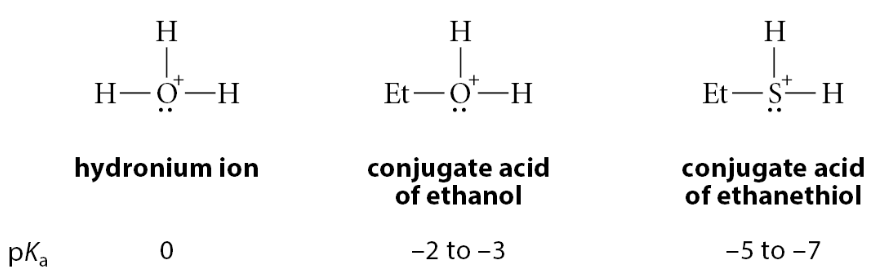
What can happen to alcohols and thiols?
Alcohols and thiols can also be protonated to form positively charged conjugate acids
Which is more basic: alcohols, water, thiols
Alcohols are slightly less basic than water; thiols, however, are much less basic
What does a negative pKa value mean?
The negative pKa values mean that protonated species are very strong acids, and that their neutral conjugate bases are rather weak
What does it mean that alcohols and thiols are amphoteric substances?
They can either gain or lose a proton. Two acid–base equilibria are associated with an alcohol:
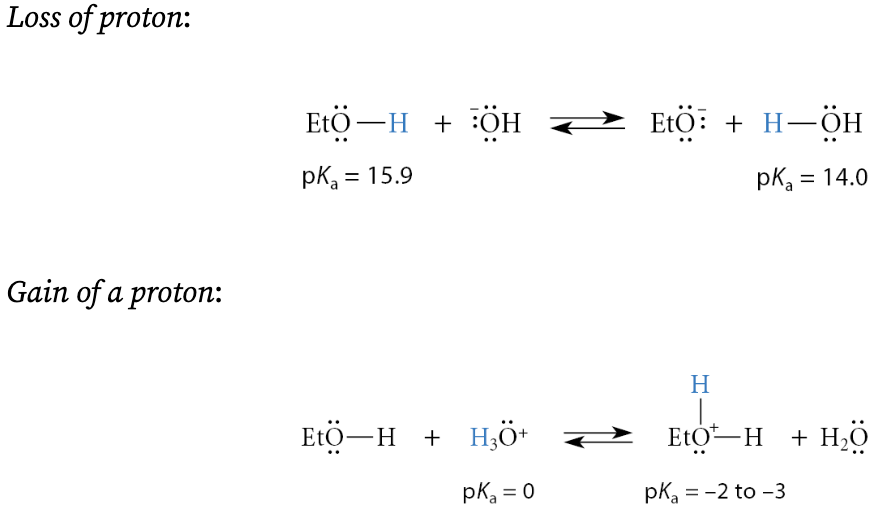
What is that acidity of an alcohols and basicity of alcohols?
Acidity of an alcohol: loss of a proton
Basicity of an alcohol: gain of a proton
Since alcohols are weak acids, when does the loss of a proton occur?
This reaction occurs usually only in the presence of strong bases
Since alcohols are weak bases, when does the gain of a proton occur?
This reaction usually occurs significantly only in the presence of strong acids
Which is more acidic: thiols or alcohols
Thiols
Which is more acidic: conjugate acids of thiols or conjugate acids of alcohols
Conjugate acids of thiols
Which is more basic: thiols or alcohols
Alcohols
11.2 Dehydration of Alcohols
What do concentrated strong acids, like H2SO4 and H3PO4, catalyze?
They catalyze a β-elimination reaction in which water is lost from a secondary or tertiary alcohol to give an alkene
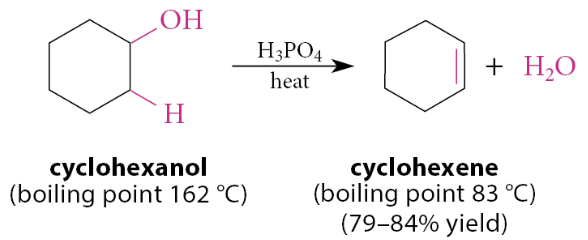
Why can alkenes be removed by distillation?
They have considerably lower boiling points than alcohols with the same carbon skeleton
What is the role of an acid catalyst in dehydration reactions?
To convert the OH group, a poor leaving group, into the O+H2 group, a good leaving group (because H2O is a weak base)
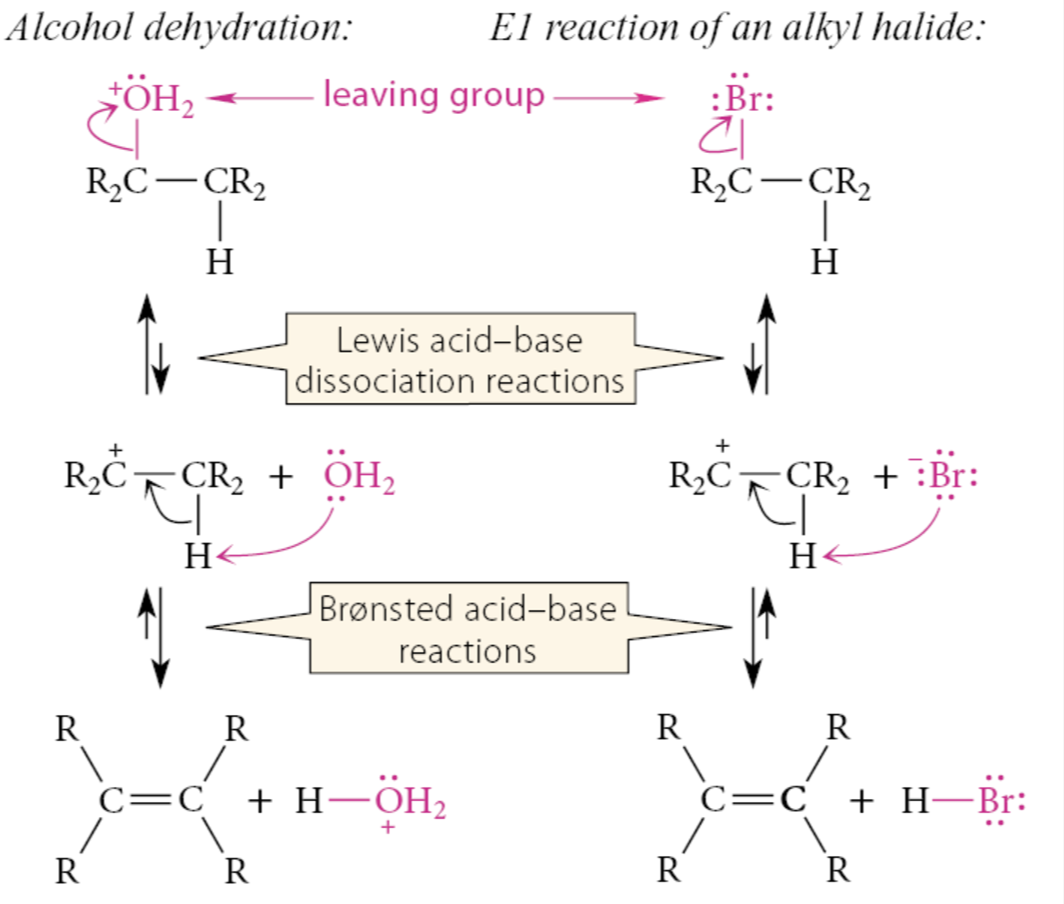
What is the first mechanistic step for alcohol dehydration?
The OH is converted into a leaving group by acting as a Brønsted base and accepting a proton from the catalyzing acid:
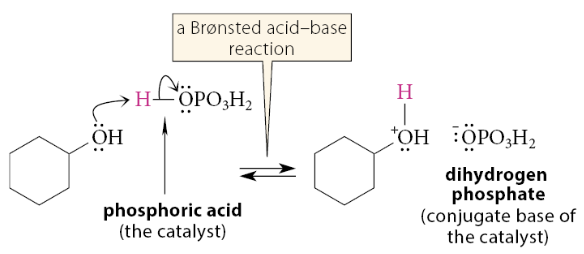
What is the second mechanistic step for alcohol dehydration?
The carbon-oxygen bond of the alcohol breaks in a Lewis acid-base dissociation to give water and a carbocation:
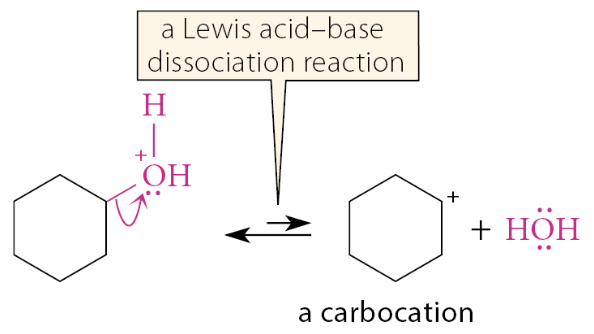
What is the third mechanistic step for alcohol dehydration?
The conjugate base OPO3H2 of the catalyzing acid removes a β-proton from the carbocation in another Brønsted acid-base reaction. This step generates the alkene product and regenerates the catalyzing acid H3PO4.
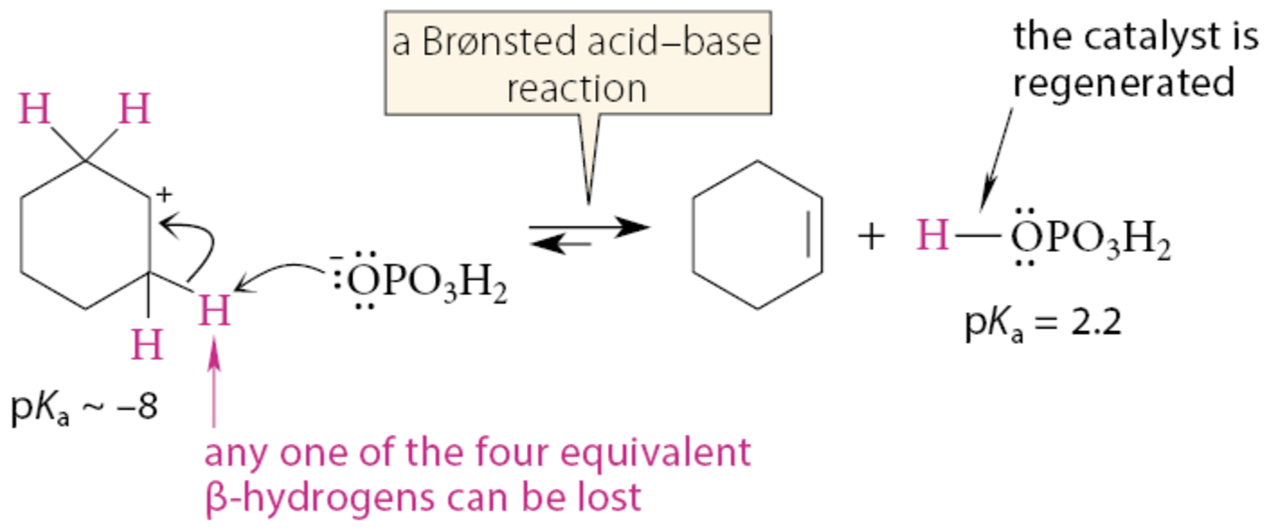
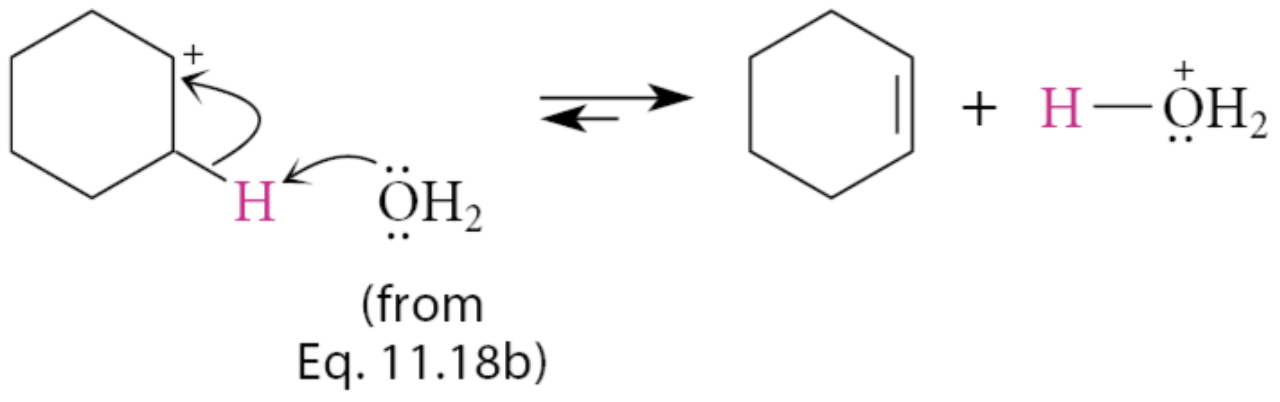
What can the H2O by-product, from the second step, or the alcohol starting material serve as?
The base that removes a β-proton from the carbocation. The H3O+ formed in this reaction can also serve as an acid catalyst in the dehydration.
Essentially, what is alcohol dehydration like?
An E1 reaction. Once the OH group of the alcohol is protonated, it becomes a very good leaving group (water). Like a halide leaving group in the E1 reaction, the protonated OH departs to give a carbocation, which then loses a β-proton to give an alkene. In alcohol dehydration, however, the rate-determining and product-determining step is the same: loss of the β-proton from the carbocation.
What is the relative rates of alcohol dehydration?
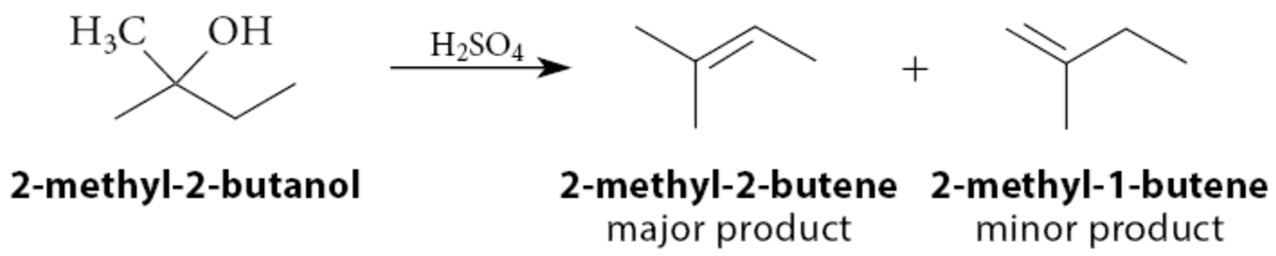
Tertiary > Secondary > Primary
What happens if an alcohol has more than one type of β-hydrogen
A mixture of alkene products can be expected. As in the E1 reaction of alkyl halides, the most stable alkene, the one with the greatest number of branches at the double bond, is the alkene formed in greatest amount:
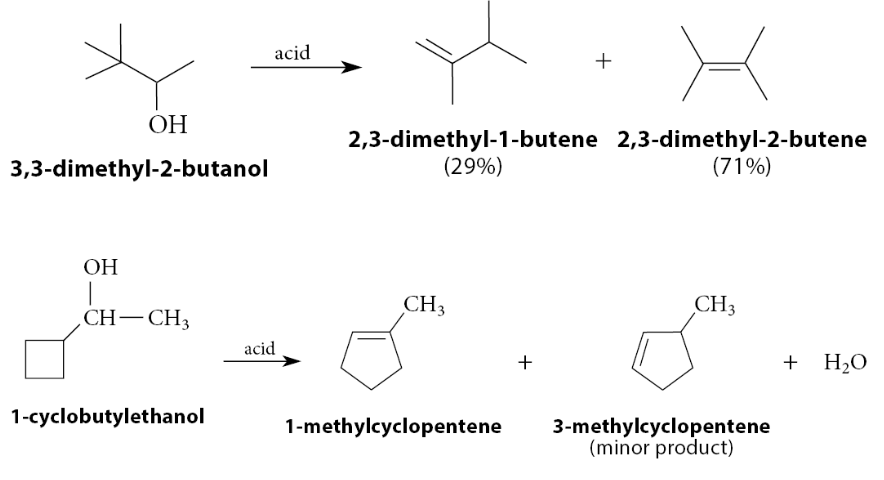
What do alcohols that react to give rearrangement-prone carbocation intermediates yield
Rearranged alkenes:
11.3 Reactions of Alcohols with Hydrogen Halides
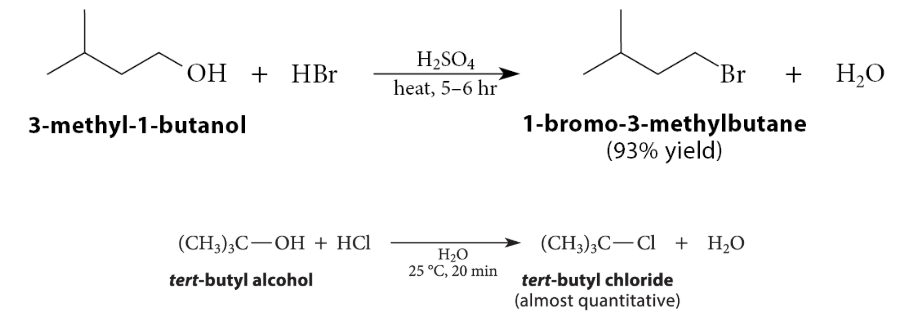
Alcohols can react with hydrogen halides to give what?
Alkyl halides
What does the mechanism of alkyl halide formation depend on?
The type of alcohol used as the starting material
What happens in the reactions of tertiary alcohols?
Protonation of the alcohol oxygen is followed by carbocation formation. The carbocation reacts with the halide ion, which is formed by ionization of strong acid HCl, and which is present in great excess:
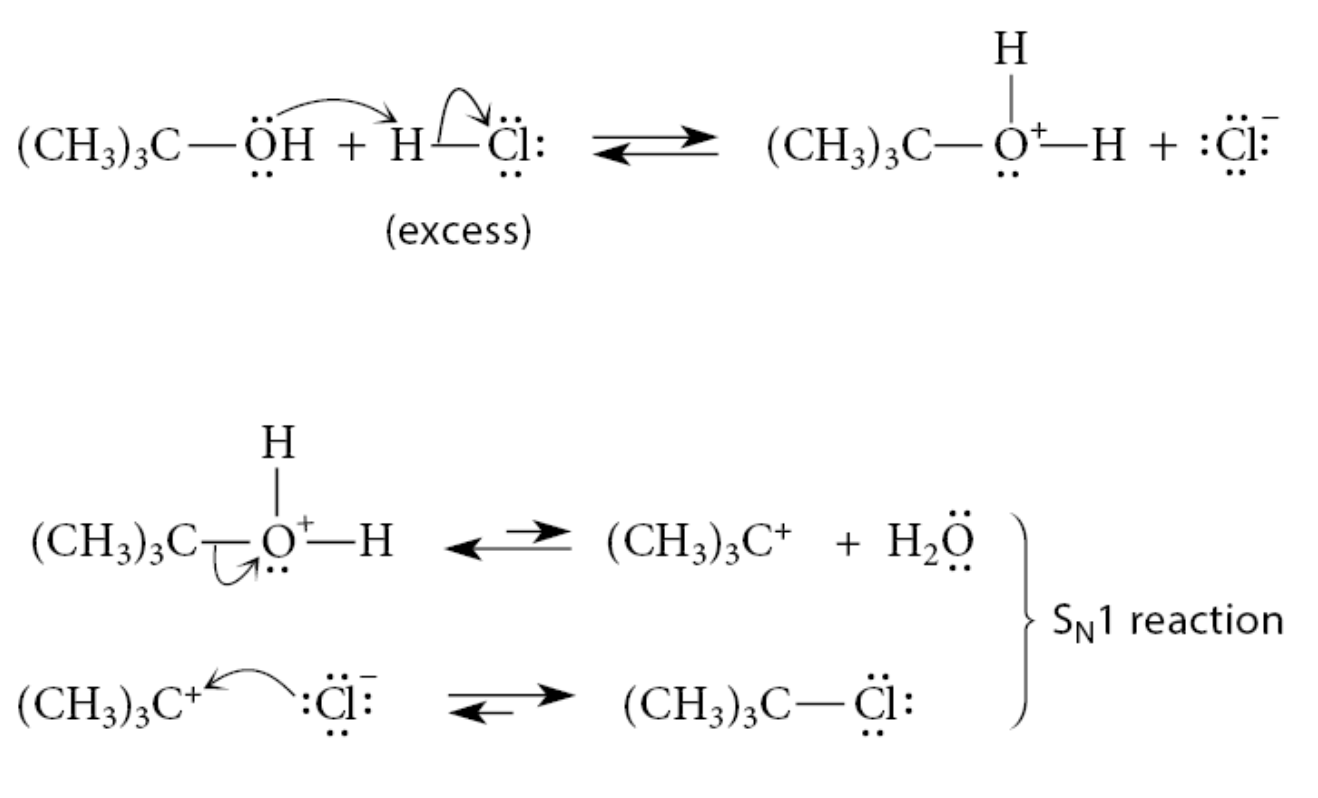
Once the alcohol is protonated, the reaction is an SN1 reaction with H2O as the leaving group.
What happens in the reactions of primary alcohols?
The reaction occurs as a concerted displacement of water from the protonated alcohol by halide ion. In other words, it is an SN2 reaction in which water is the leaving group.


Notice that the initial step in both the SN1 and the SN2 mechanism is protonation of the OH group.
At what room temperatures do tertiary alcohols and primary alcohols react in with hydrogen halides?
Tertiary alcohols react with hydrogen halides rapidly at room temperature, whereas the reactions of primary alcohols require heating for several hours.
The reactions of primary alcohols with HBr and HI are satisfactory, but their reactions with what make it very slow?
HCl
What happens in the reactions of secondary alcohols?
The reactions tend to occur by the SN1 mechanism. This means that carbocations are involved as reactive intermediates; consequently, rearrangements can occur, as in the following example:
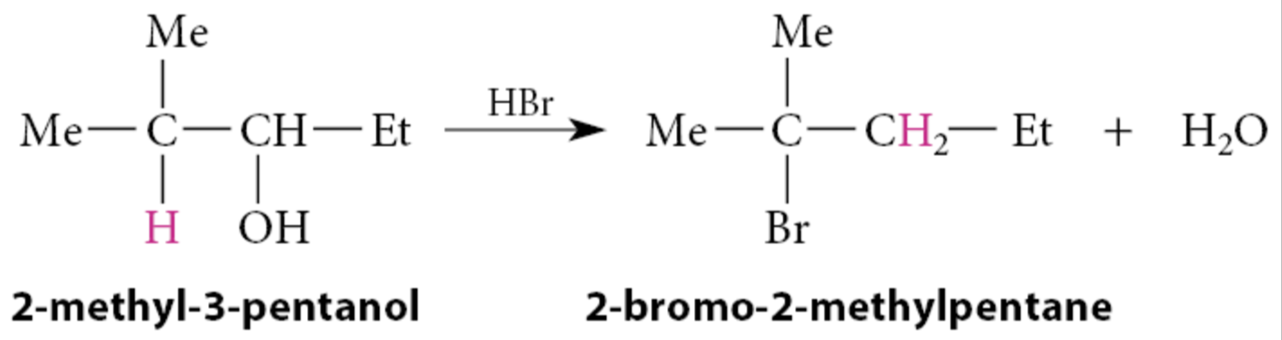
What is common between dehydration of alcohols to alkenes and the reactions of alcohols with hydrogen halides?
Both take place in very acidic solution; in both reactions, the acid converts the OH group into a good leaving group
What happens in substitution reactions if acid were not present?
The halide ion would have to displace OH to form the alkyl halide. This reaction does not take place because OH is a much stronger base than any halide ion, and strong bases are poor leaving groups
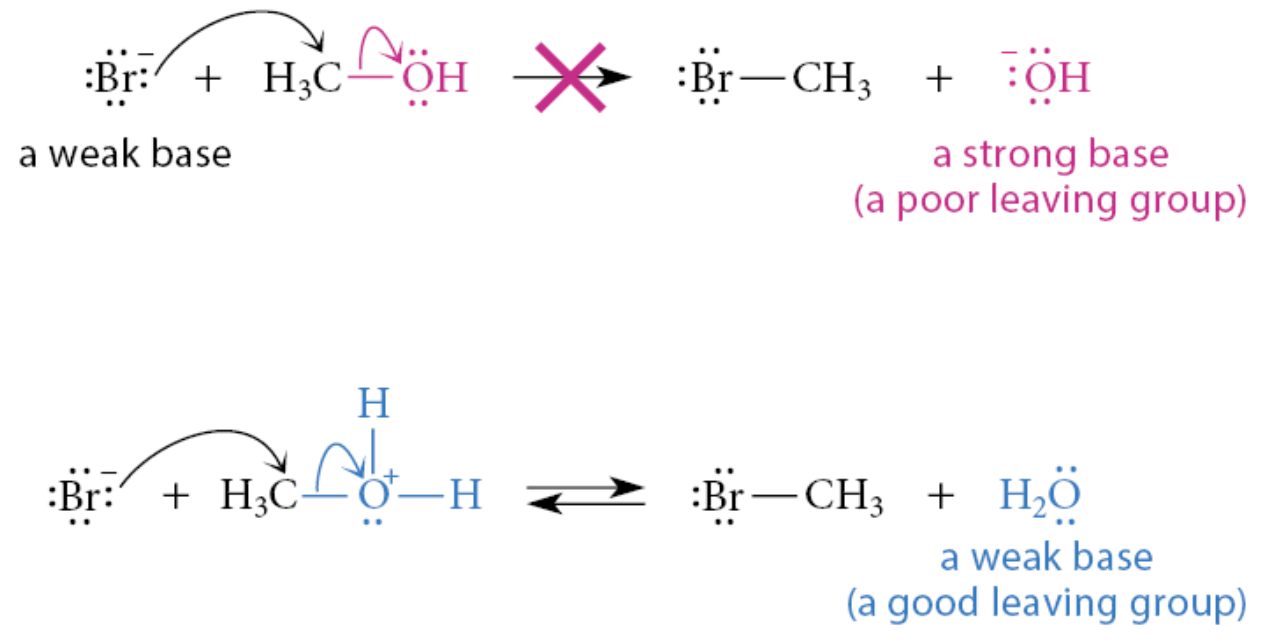
What is the initial step for the formation of secondary and tertiary alkyl halides and the dehydration of secondary and tertiary alcohols?
Protonation of the alcohol oxygen and formation of a carbocation
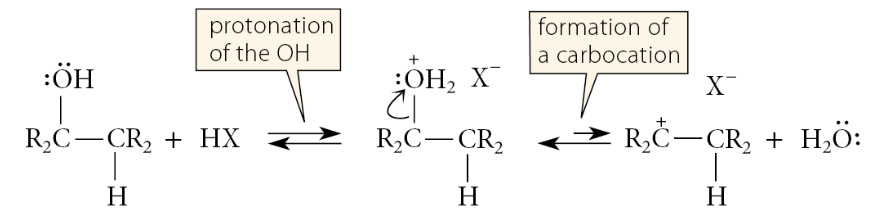
What happens to the fate of the carbocation in formation of alkyl halides and the dehydration of alcohols?
In the presence of a hydrogen halide, the halide ion is present in excess and reacts with the carbocation to give an alkyl halide, which (if the solvent is aqueous acid) comes out of solution. In dehydration, no halide ion is present, and when the alkene forms by loss of a β-proton from the carbocation, the conditions of the dehydration reaction force the removal of the alkene product and the water by-product from the reaction mixture. It follows, then, that alkyl halide formation and dehydration to alkenes are alternative branches of a common mechanism:

11.4 Alcohol-Derived Leaving Groups
What is an important method of activating alcohols toward nucleophilic substitution and β-elimination reactions
Convert them into sulfonate esters, derivatives of sulfonic acids
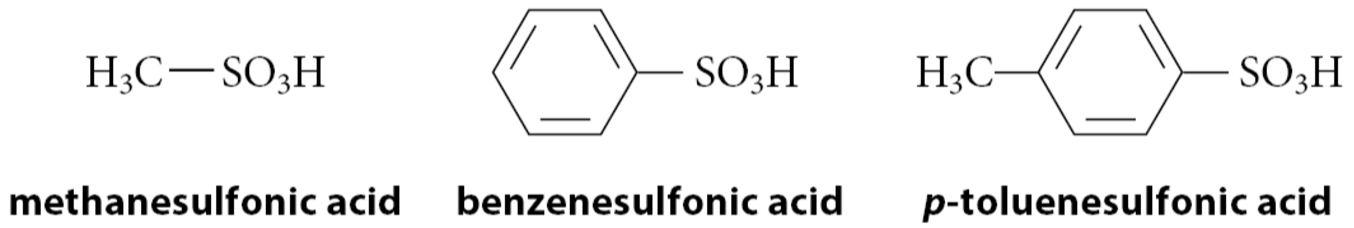
What are sulfonic acids?
Compounds of the form R—SO3H
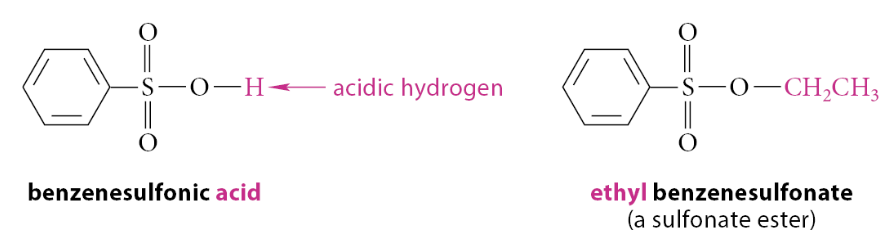
What are sulfonate esters?
A compound in which the acidic hydrogen of a sulfonic acid is replaced by an alkyl or aryl group. For example, in ethyl benzenesulfonate, the acidic hydrogen of benzenesulfonic acid is replaced by an ethyl group
What are mesylates and tosylates?
Esters of methanesulfonic acid are called mesylates (abbreviated R—OMs), and esters of p-toluenesulfonic acid are called tosylates (abbreviated R—OTs)

How are sulfonate esters formed?
Sulfonate esters are prepared from alcohols and other sulfonic acid derivatives called sulfonyl chlorides. For example, p-toluenesulfonyl chloride (also known as tosyl chloride, TsCl) is the sulfonyl chloride used to prepare tosylate esters
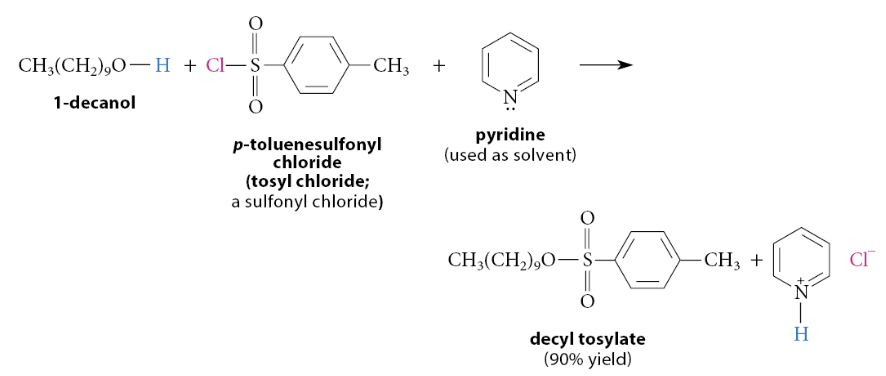
This is a nucleophilic substitution reaction in which the oxygen of the alcohol displaces chloride ion from the tosyl chloride. The pyridine used as the solvent is a base. Besides catalyzing the reaction, it also neutralizes the HCl that would otherwise form in the reaction
Why are sulfonate esters helpful?
They have approximately the same reactivities as the corresponding alkyl bromides in substitution and elimination reactions. The reason for this similarity is that sulfonate anions, like bromide ions, are good leaving groups. Good leaving groups are weak bases. Sulfonate anions are weak bases because they are the conjugate bases of sulfonic acids, which are strong acids
Sulfonate ester prepared from primary and secondary alcohols undergo what reaction?
SN2
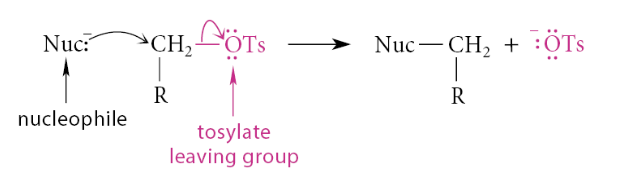
What reaction do secondary and tertiary sulfonate esters undergo?
E2 reactions with strong bases and they undergo SN1–E1 solvolysis reactions in polar protic solvents.
What is a triflate group?
Occasionally we might need a sulfonate ester that is much more reactive than a tosylate or mesylate. In such a case a trifluoromethanesulfonate ester is used. The trifluoromethanesulfonate group is nicknamed the triflate group and is abbreviated OTf
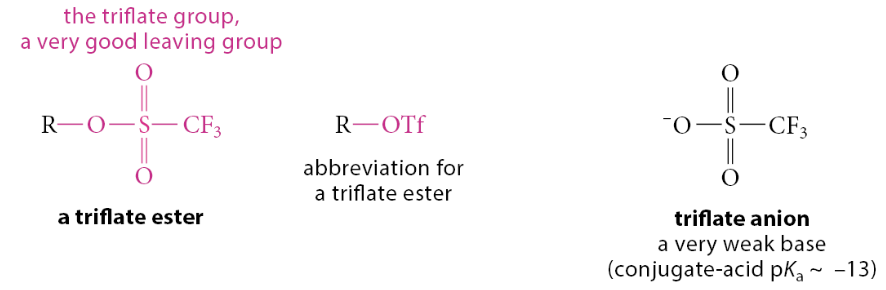
Is a triflate anion a weak or strong base?
Weak base, making it an exceptionally good leaving group, and triflate esters are highly reactive
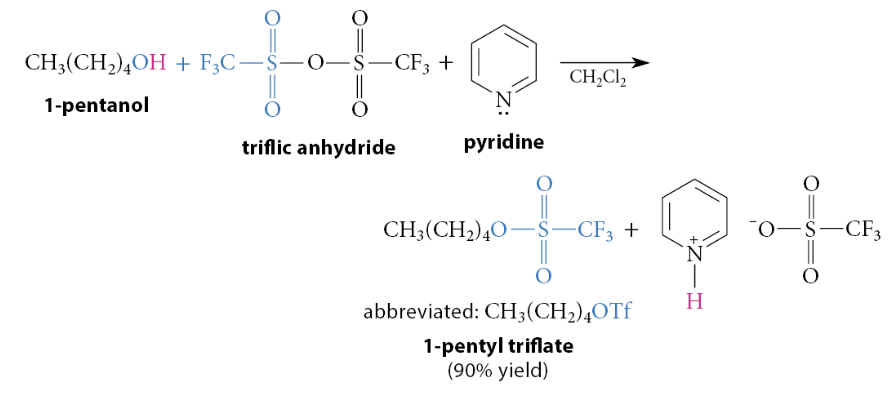
How are triflate esters prepared?
Triflate esters are prepared in the same manner as tosylate esters, except that triflic anhydride is used instead of tosyl chloride
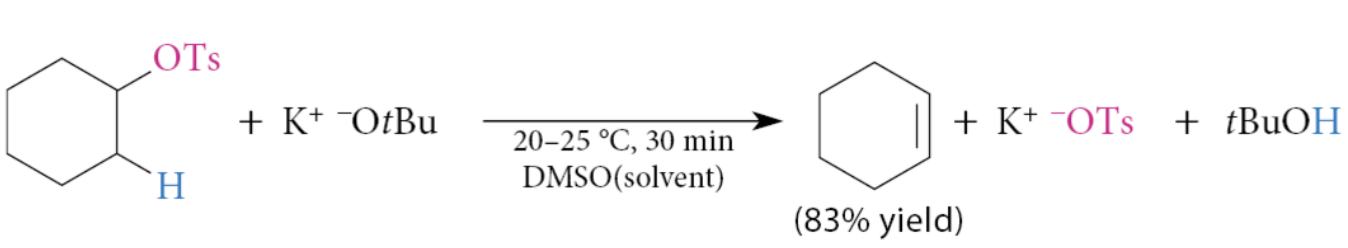
What can the E2 reactions of sulfonate esters be used to prepare?
Alkenes
This reaction is especially useful when the acidic conditions of alcohol dehydration lead to rearrangements or other side reactions, or for primary alcohols in which dehydration is not an option.
An alcohol can be made to undergo what reactions?
Substitution and elimination reactions typical of the corresponding alkyl halides by converting the OH group into a good leaving group such as a sulfonate ester.
What does alkylation refer to?
In this reaction, the nucleophile gets an alkyl group from the alkyl halide or sulfonate ester, similar to how a Brønsted base gets a proton from an acid. Because of this, alkyl halides, sulfonate esters, and similar compounds with good leaving groups are called alkylating agents. A good alkylating agent reacts quickly with nucleophiles in SN2 or SN1 reactions to transfer an alkyl group

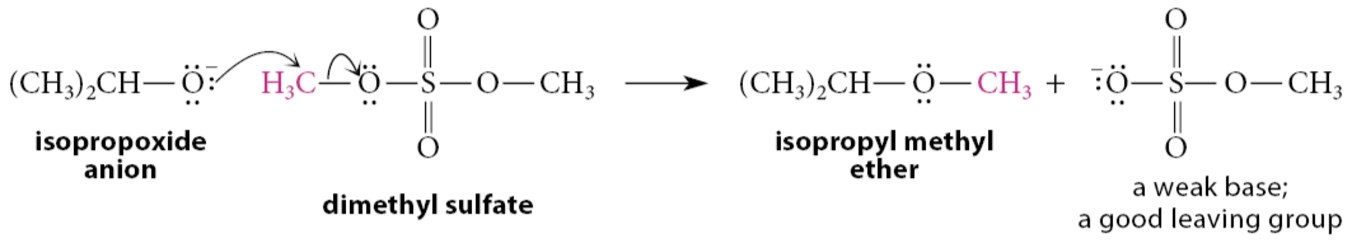
What are alkyl esters of strong inorganic acids?
Typically potent alkylating agents because they contain leaving groups that are very weak bases. For example, dimethyl sulfate is an effective methylating agent, as shown in the following example
What is a better method for the preparation of primary alkyl chlorides?
The reaction of alcohols with thionyl chloride

What does the preparation of an alkyl chloride from an alcohol with thionyl chloride?
Involves the conversion of the alcohol OH group into a good leaving group. When an alcohol reacts with thionyl chloride, a chlorosulfite ester intermediate is formed

What happens to the chlorosulfite ester?
It reacts readily with nucleophiles because the chlorosulfite group, —O—SO—Cl, is a very weak base and therefore a very good leaving group. The chlorosulfite ester is usually not isolated, but it reacts with the chloride ion formed to give the alkyl chloride. The displaced −O—SO—Cl ion is unstable and decomposes to SO2 and Cl−

Can thionyl chloride method also be used for secondary alcohols?
it can also be used with secondary alcohols, although rearrangements in such cases have been known to occur. Rearrangements are best avoided in the preparation of secondary alkyl halides by using SN2 conditions: the reaction of a halide ion with a sulfonate ester in a polar aprotic solvent
What is a related method for the conversion of alcohols into alkyl bromides
Involves the use of Ph3PBr2 (dibromotriphenylphosphorane or triphenylphosphine dibromide)

What are the steps for using Ph3PBr2?
The first mechanistic step of the reaction is an SN2 reaction in which the oxygen of the alcohol acts as a nucleophilic center and phosphorus as the electrophilic center. The bromide ion of the reagent then acts as a base to give HBr and an intermediate:

The bromide ion that was displaced then acts as a nucleophile at the α-carbon of the intermediate to displace triphenylphosphine oxide as a leaving group, giving the product alkyl halide

What can the reaction of alcohols with triphenylphosphine dibromide even be carried out successfully with?
Neopentyl alcohol. Recall that neopentyl derivatives are unreactive in SN2 reactions

What is the triphenylphosphine dibromide reaction is particularly useful for the preparation of?
Secondary bromides
11.5 Conversion of Alcohols into Alkyl Halides: Summary
What are the variety of reactions that can be used to convert alcohols into alkyl halides?
Reaction with hydrogen halides
Formation of sulfonate esters followed by SN2 reaction with halide ions
Reaction with thionyl chloride (SOCl2) or triphenylphosphine dibromide (Ph3PBr2)
Which method should be used for primary alcohols?
Alkyl bromides are prepared from primary alcohols by the reaction of the alcohol with concentrated HBr or with Ph3PBr2. HBr is often chosen for convenience and because the reagent is relatively inexpensive. The reaction with Ph3PBr2 is quite general, but it is particularly useful when the alcohol contains another functional group that would be adversely affected by the strongly acidic conditions of the HBr reaction. Primary alkyl iodides can be prepared with HI, which is usually supplied by mixing an iodide salt such as KI with a strong acid such as phosphoric acid. Thionyl chloride is the method of choice for the preparation of primary alkyl chlorides because the reactions of primary alcohols with HCl are slow. The sulfonate ester method works well with primary alcohols, but it requires two separate reactions (formation of the sulfonate ester, then reaction of the ester with halide ion). Because all of these methods have an SN2 mechanism as their basis, alcohols with several β-alkyl substituents, such as neopentyl alcohol, do not react with any of these reagents except Ph3PBr2. This reagent does work with such alcohols because it is accelerated
Which method should be used for tertiary alcohols?
Tertiary alcohols react rapidly with HCl or HBr under mild conditions to give the corresponding alkyl halides. The sulfonate ester method is not used with tertiary alcohols because tertiary sulfonates, like tertiary alkyl halides, do not undergo SN2 reactions.
Which method should be used for secondary alcohols?
If the secondary alcohol has no β-alkyl substitution, the thionyl chloride method can be used to prepare alkyl chlorides. To avoid rearrangements completely, the alcohol can be converted into a sulfonate ester, which can be treated, in turn, with the appropriate halide ion (Cl−, Br−, or I−) in a polar aprotic solvent. This type of solvent provides the enhanced nucleophilicity of the halide ion necessary to overcome the relatively low SN2 reaction rate of a secondary sulfonate ester. Less reactive secondary alcohols can be converted into triflates, which are much more reactive than tosylates or mesylates toward halide ions in polar aprotic solvents. The HBr method can be expected to lead to rearrangements and is therefore not satisfactory (unless rearranged products are desired). The Ph3PBr2 method can be used to form alkyl bromides without rearrangement from primary and secondary alcohols that have significant β-alkyl substitution.
What are two general strategies to make the —OH group must a good leaving group?
Protonation: Protonated alcohols are intermediates in both the dehydration to alkenes and the reaction with hydrogen halides to give alkyl halides.
Conversion into sulfonate esters, inorganic esters, or related leaving groups: Sulfonate esters, to a useful approximation, react like alkyl halides. That is, the principles of alkyl halide reactivity covered in Chapter 9 are equally applicable to sulfonate esters. Thionyl chloride and triphenylphosphine dibromide are additional examples of this approach in which the reagent both converts the alcohol —OH into a good leaving group and provides the displacing nucleophile.
11.6 Oxidation and Reduction in Organic Chemistry
What does oxidation and reduction refer to?
Oxidation is a transformation in which electrons are lost
Reduction is a transformation in which electrons are gained
What is each oxidation accompanied by?
A reduction and vice versa
What is a half-reaction?
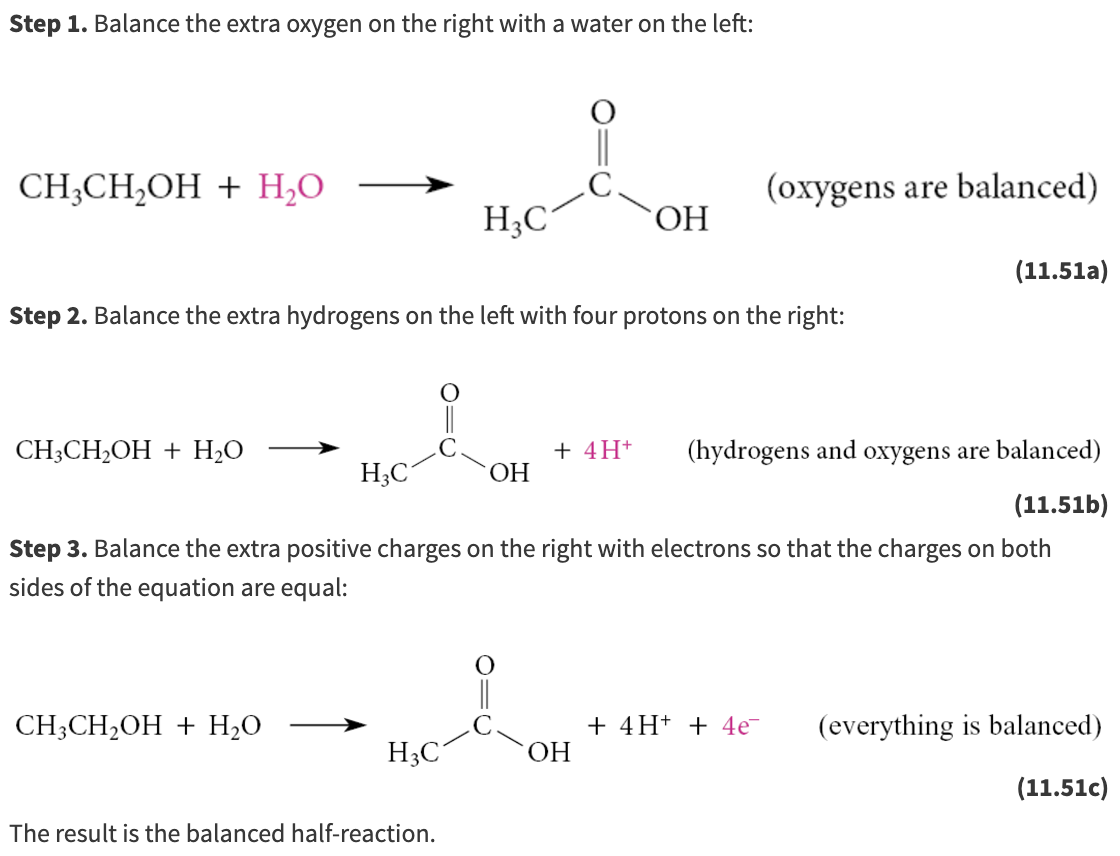
The gain or loss of electrons can be illustrated with a half-reaction, which shows either the oxidation or the reduction but not both. An example of such a half-reaction in organic chemistry is the oxidation of ethanol to acetic acid:

This is a half-reaction because the reagent that brings about this oxidation (and is itself reduced) is not included. That this is an oxidation can be demonstrated by balancing the half-reaction using protons and free electrons
What are the steps for half-reactions?
Step 1. Use H2O to balance missing oxygens.
Step 2. Use protons (that is, H+) to balance missing hydrogens.
Step 3. Use electrons to balance charges.