Orbitals Hybridization and Biological Reactivity
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
22 Terms
Orbitals hybridization
Mixing atomic orbitals to form new hybrid orbitals.
Hybrid orbitals
Created to increase valence number for reactivity.
Expanded valence
Higher number of available bonding electrons.
Molecular geometry
Shape of molecules determined by orbital properties.
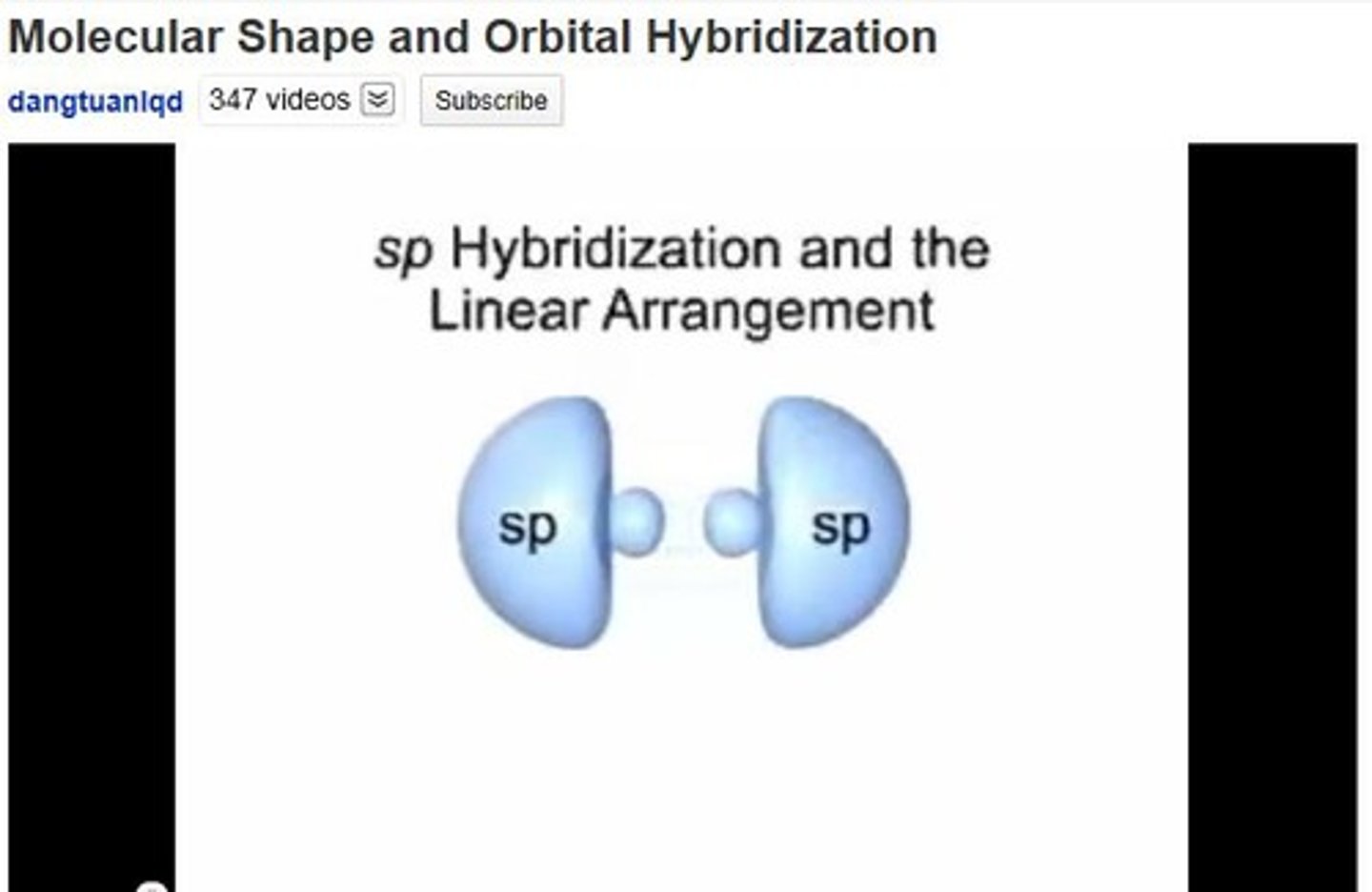
sp hybridization
One s and one p orbital combine; example: BeCl2.
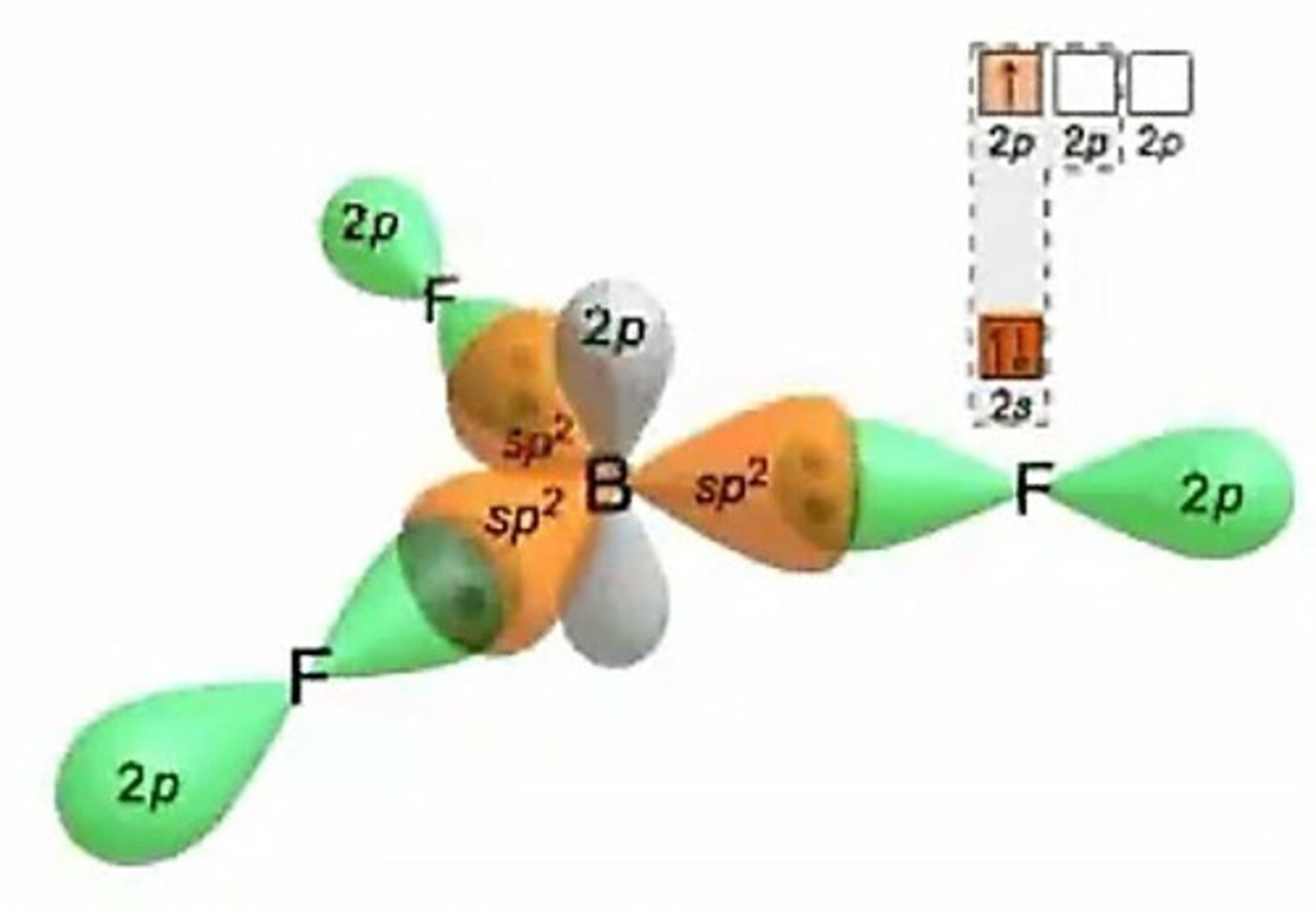
sp2 hybridization
One s and two p orbitals combine; example: BF3.
sp3d hybridization
Three p and one d orbital combine; example: P.
Phosphorus (P)
Can share five covalent bonds due to hybridization.
Phosphate (PO4)
Essential for linking nucleotides in DNA/RNA.
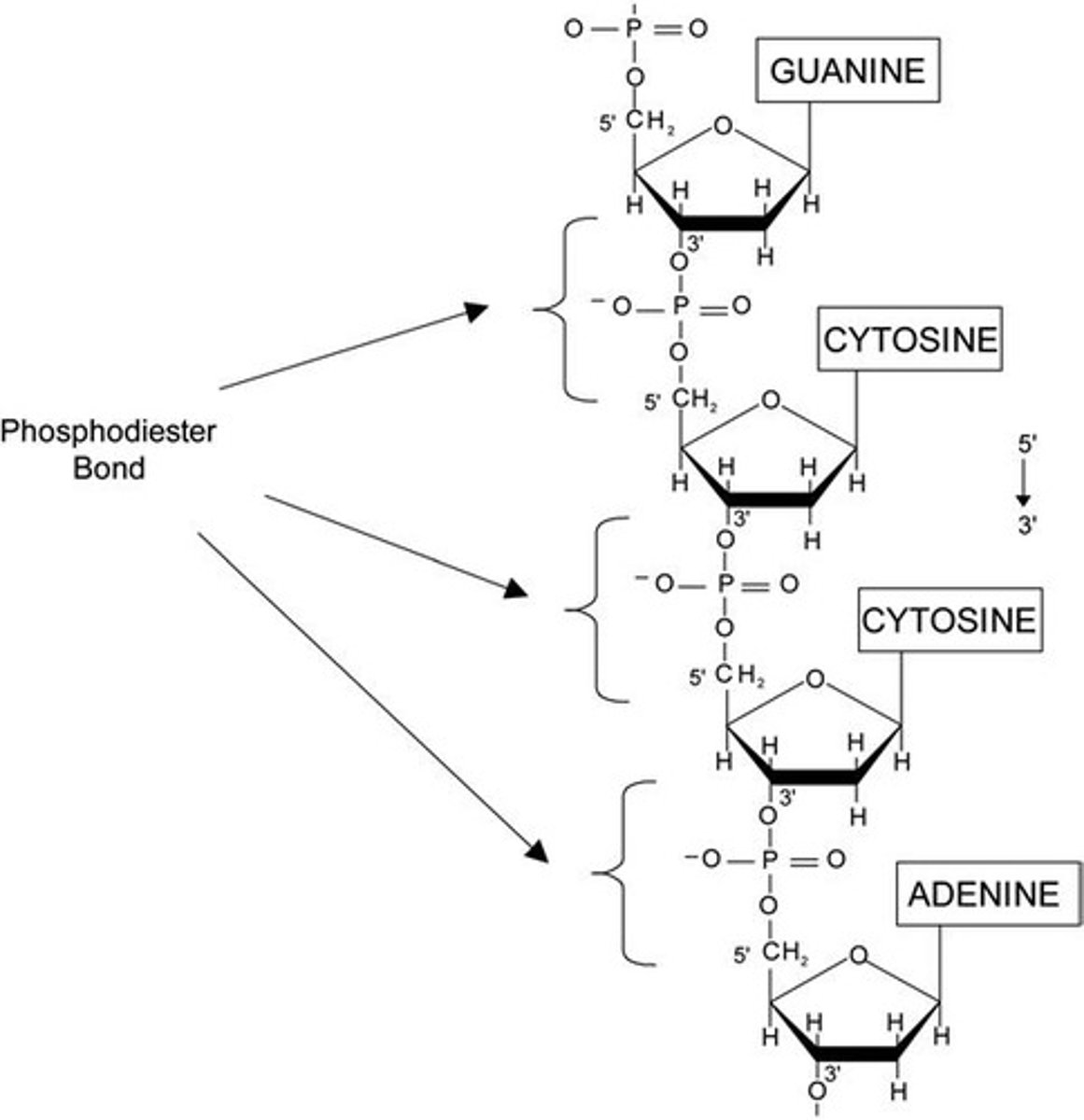
Phosphodiester bond
Covalent bond linking nucleotides in nucleic acids.
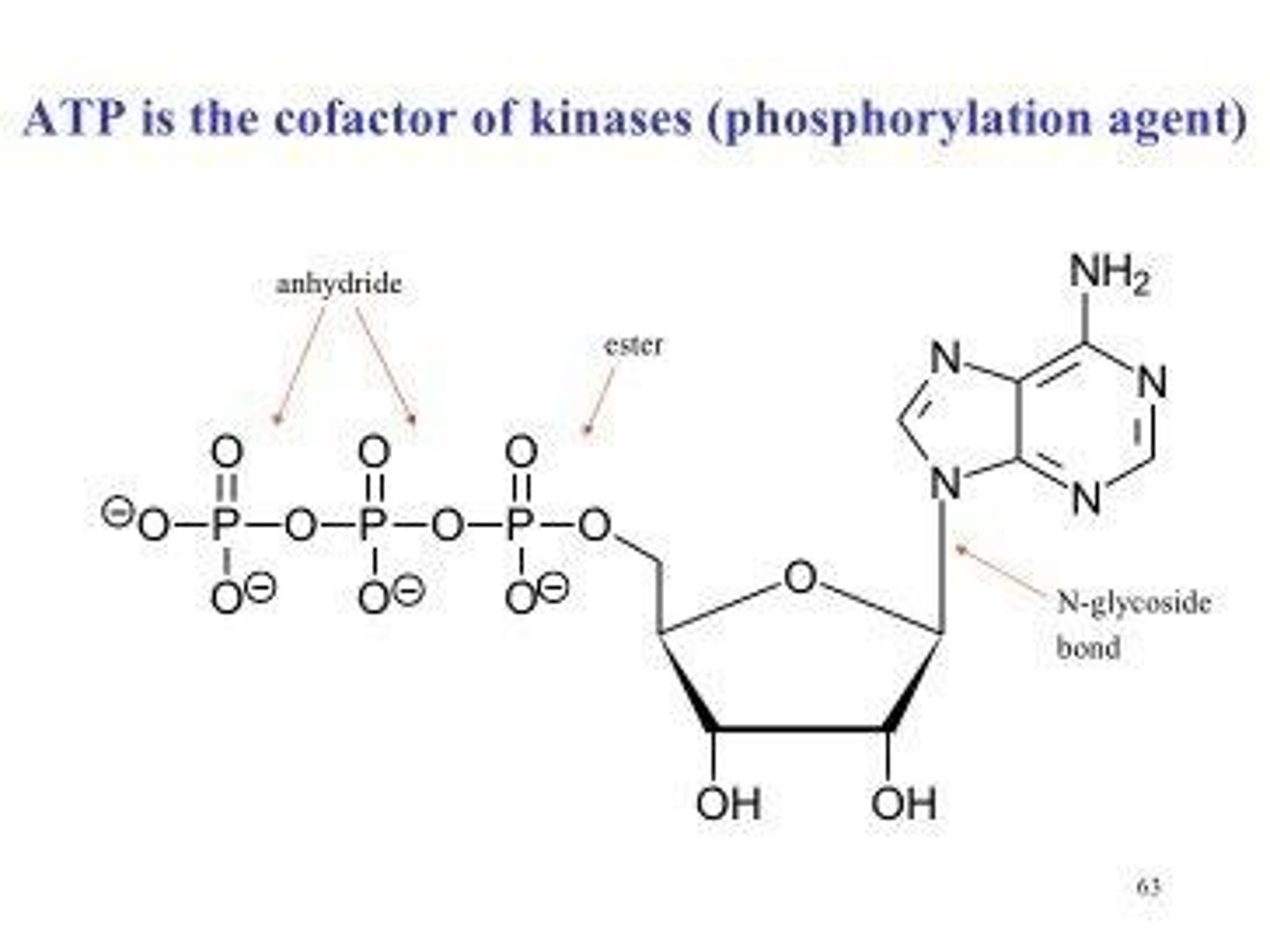
Adenosine triphosphate (ATP)
Main energy currency in biological processes.
Anhydride bond
Type of bond in ATP providing energy.
Carbon (C)
Can undergo sp, sp2, or sp3 hybridization.
Chemical versatility
Carbon's ability to form diverse compounds.
Hydroxyapatite crystals
Calcium and phosphorus compound in bones.
Valence electrons
Electrons available for bonding in an atom.
Ground state
Lowest energy state of an atom's electrons.
Aufbau principle
Electrons fill orbitals starting from lowest energy.
Covalent bonds
Bonds formed by sharing electron pairs.
Biological processes
Life functions dependent on chemical reactions.
Reactivity of elements
Influenced by orbital hybridization and valence.
Unique hybridization
Allows elements like carbon to form various compounds.