4.8 The Structure and Properties of Solids
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What are the four types of solids?
Ionic Solids: metal and nonmetal
Metallic Solid: two metals
Molecular Solid: two nonmetals
Covalent Network: metalloids/carbon
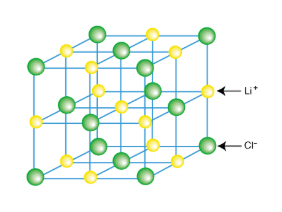
Ionic Crystals
Anions and cations are attracted together in CRYSTAL LATTICE
Each anion surrounded by cations and vice versa
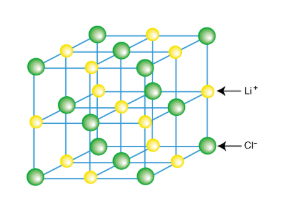
Crystal Lattice
Exists in three dimension
Ionic substance held together by STRONG electrostatic attractions in all three dimensions
Bonds are directional
Lattice is composed of ions
No molecules in ionic compounds, referred to as formula units
Physical Properties of Ionic Compounds
hard, brittle crystalline solids
relatively high melting and boiling points
do not conduct electricity when solid
conduct electricity when molten or in aqueous solution
soluble in water
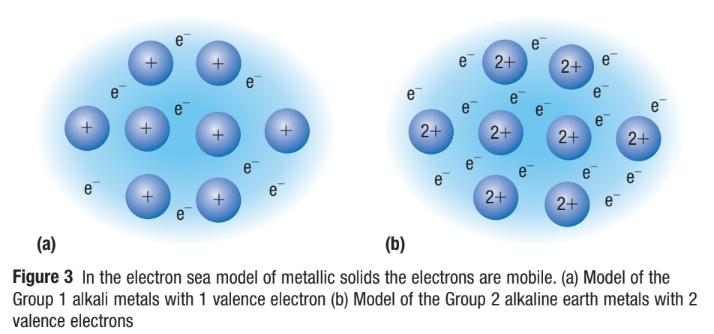
Metallic Crystals
Atoms are packed closely together in 3 dimensions (CLOSE-PACKED LATTICE)
Metals have low ionization energies and low energy unfilled orbitals
So the valence electrons become DELOCALIZED amongst (shared by) all the atoms
No electrons belong to a particular atom, free to move throughout metal
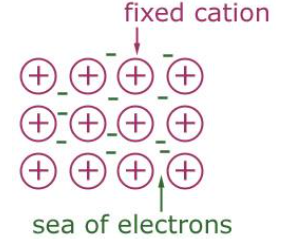
Metallic Bonding
Atoms have lost valence electrons, are positively charged (CATIONS)
attraction of positive ions for mobile electrons provides force that holds structure together
a lattice of positive ions filled by a mobile ‘sea’ of valence electrons
Metals consist of closely packed atoms with free-moving valence electrons.
The positively charged nuclei remain fixed while the electrons move between them.
Metals are held together by metallic bonding, where electrons move freely between nuclei.
“Sea of electrons” results in different physical properties of metallic crystals
Physical Properties of Metals
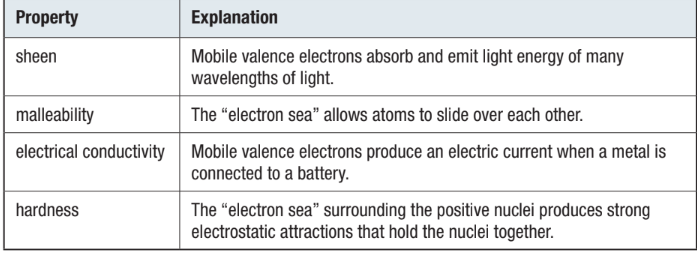
attraction is between ions and mobile electrons, not between ions themselves
this means that layers of ions can slide past each other without breaking any bonds
means that metals are MALLEABLE and DUCTILE
if atoms are different sizes (in ALLOYS), it is harder for layers to slide
alloys are usually harder than pure metals
delocalized electrons are free to move from one side of lattice to the other and can carry an electric current
metals are good CONDUCTORS of both electricity and heat
strength of bond between metals depends on how many electrons each atom shares
MP of Potassium is 337 K
MP of Calcium is 1123 K
MP of Scandium is 1703 K
strength also depends on how far from the nucleus the sea of electrons are going down a group, the melting points will decrease
Table of Metallic Properties
Molecular Crystals
The intermolecular forces (weaker than intramolecular forces) determine the structure and properties of molecular crystals.
Types of intermolecular forces:
London dispersion forces
Dipole-dipole forces
Hydrogen bonding (especially in polar molecules)
Polar molecules have both dipole-dipole and London dispersion forces.
Non-polar molecules only have London dispersion forces.
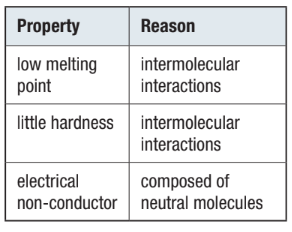
Properties of Molecular Crystals:
Due to weak intermolecular forces, molecular crystals tend to:
Have lower melting points.
Be less hard than ionic crystals.
Molecular crystals contain neutral molecules, so they do not conduct electricity well, either in pure form or in solution.
Properties of Molecular Crystals Table
Covalent Network Solids
A covalent network crystal is a solid where covalent bonds form an interwoven network between atoms.
Giant, three dimensional covalent structure
The network of covalent bonds in these crystals contributes to their unique properties, such as hardness and strength.
Look at the allotropes of carbon and at silicon dioxide
ALLOTROPES: different forms of an element that exist in the same physical state
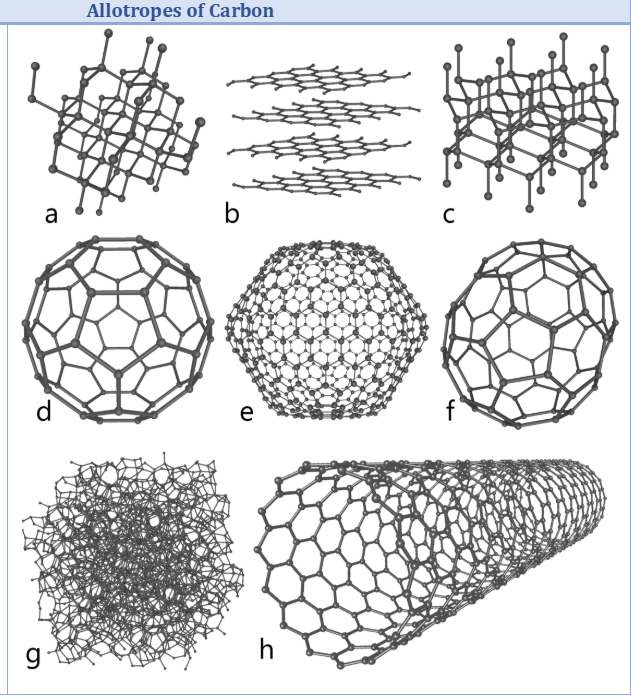
Diamond
Each carbon is joined to 4 other carbons in the tetrahedral shape
Extremely strong structure!
All intramolecular bonds
Explains high MP, BP and exceptionally hard structure
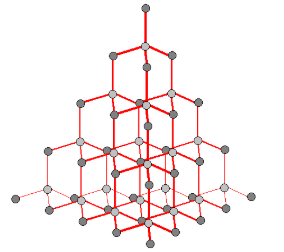
Graphite
comprises a giant covalent network in 2 dimensions
each layer has C atoms bonded to 3 other C atoms
each layer is very strong
between the layers, only weak van der Waals forces hold the layers together
distance between the sheets is quite large and the forces between very weak
so layers can slide over each other easily often used as lubricant
layers are easily rubbed off on paper - why it is used in ‘lead’ pencils
there are delocalized electrons between layers that are free to move and so graphite can conduct electricity
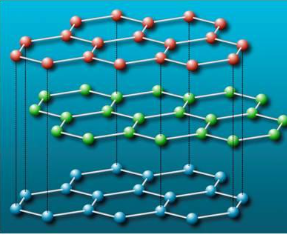
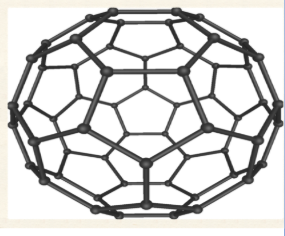
Fullerenes
approximately spherical molecule made up of five- and six-membered C ring
C60 resembles a soccer ball
there is a small amount of delocalized electrons but not enough to strongly conduct electricity
behaves as an electron deficient molecule and readily accepts electrons
is a molecular molecule and can dissolve in some non-polar solvents
lower MPs than diamond or graphite
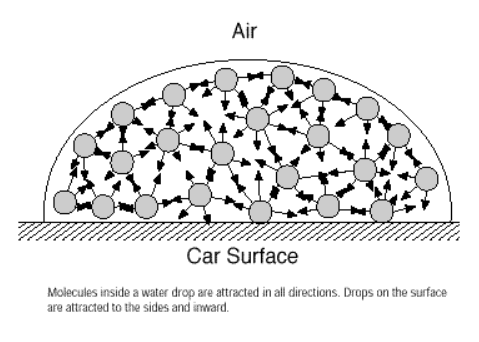
Surface Tension
surface tension on a liquid is like an elastic skin
molecules within a liquid are attracted by molecules on all sides, but molecules right at the surface are only attracted downward and sideways
in order to break the surface tension, the force must be greater than the intermolecular forces holding the molecules together
stronger forces = higher surface tensions
water has one of the highest surface tensions