CHEMISTRY TOPIC 1- Atomic Structure and the Periodic Table
1/32
Earn XP
Description and Tags
Topic 1 of Chemistry, in paper 1 of AQA
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Protons
+1 charge
+1 mass
Found in the nucleus of an atom
Discovered by Ernest Rutherford in 1919
Electrons
-1 charge
+0 mass (negligible)
Found in the electron shells
Discovered by J.J. Thomson in 1897
Neutrons
+0 charge
+1 mass
Found in the nucleus of an atom
Discovered by James Chadwick in 1932
Radius of a nucleus
1×10^-14 metres
Overall charge of a normal atom
0- neutral since the number of protons is the same as the number of electrons
Atomic number
Aka proton number
Bottom number
Tells how many protons there are
Mass number
Top number
Mass of an element
Sum of the number of protons and neutrons
Isotope
Atom of the same element but with a different number of protons
Same atomic number
Different mass number due to different amount of neutrons
Relative Atomic Mass equation (Ar)
sum of (isotope abundance * isotope mass number) / sum of abundances of all isotopes
Element
Compound
Mixture
Substance of only one atom type
Substance of 2+ different atoms chemically bonded
Substance of 2+ different atoms together with no chemical bonds
Diatomic
Element that exists as two atoms bonded together
Inert
Chemically unreactive
Soluble and Insoluble
Soluble substances dissolve in water
Insoluble substances don’t dissolve in water
Chromatography Practical Method
Draw line with pencil near bottom of filter paper (pencil is insoluble)
Add ink spot on pencil line and put bottom of sheet in beaker of solvent (water, ethanol…)
Put lid on container (prevent evaporation)
Solvent will travel up the paper, bringing and separating the ink into its components based on their different affinities to the paper (insoluble dyes in the ink will stay on pencil line)
Take paper out of beaker once the solvent is nearly at the top
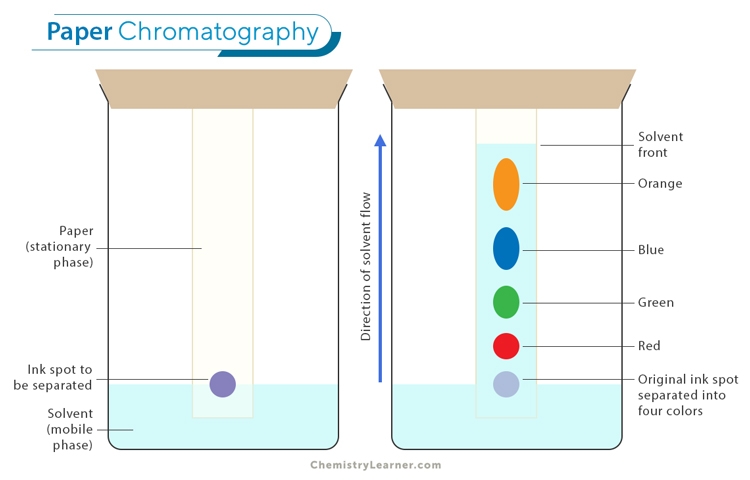
Stationary and Mobile Phase in Chromatography
Stationary- Chromatography paper
Mobile- Solvent that moves through stationary phase and carries mixture
Filtration
Separate an insoluble solid from a liquid mixture
Also used in purification in removing solid impurities
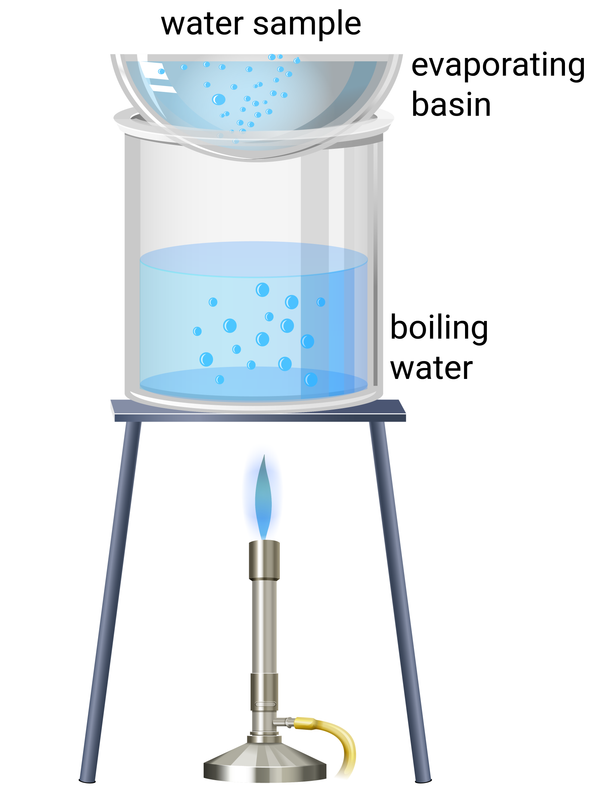
Evaporation to Separate Soluble Solid from Solution Method
Pour solution into evaporating dish on tripod with gauze mat
Heat dish using bunsen burner below (or with beaker of boiling water heated by bunsen burner) - solvent will evaporate and solution will increase in concentration
Eventually, crystals will form. Keep heating the dish until only dry crystals remain
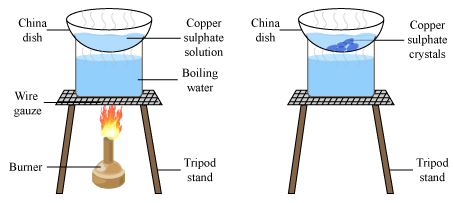
Crystallisation to Separate Soluble Solids from Solution Method
Pour solution into evaporating dish and gently heat (some will evaporate and increase concentration)
Once some is evaporated OR crystals start to form, remove dish from heat and leave solution to cool
The salt should form crystals as it becomes insoluble in cold, highly concentrated solution
Filter crystals out of solution and leave them in a warm place to dry (drying oven, desiccator…)
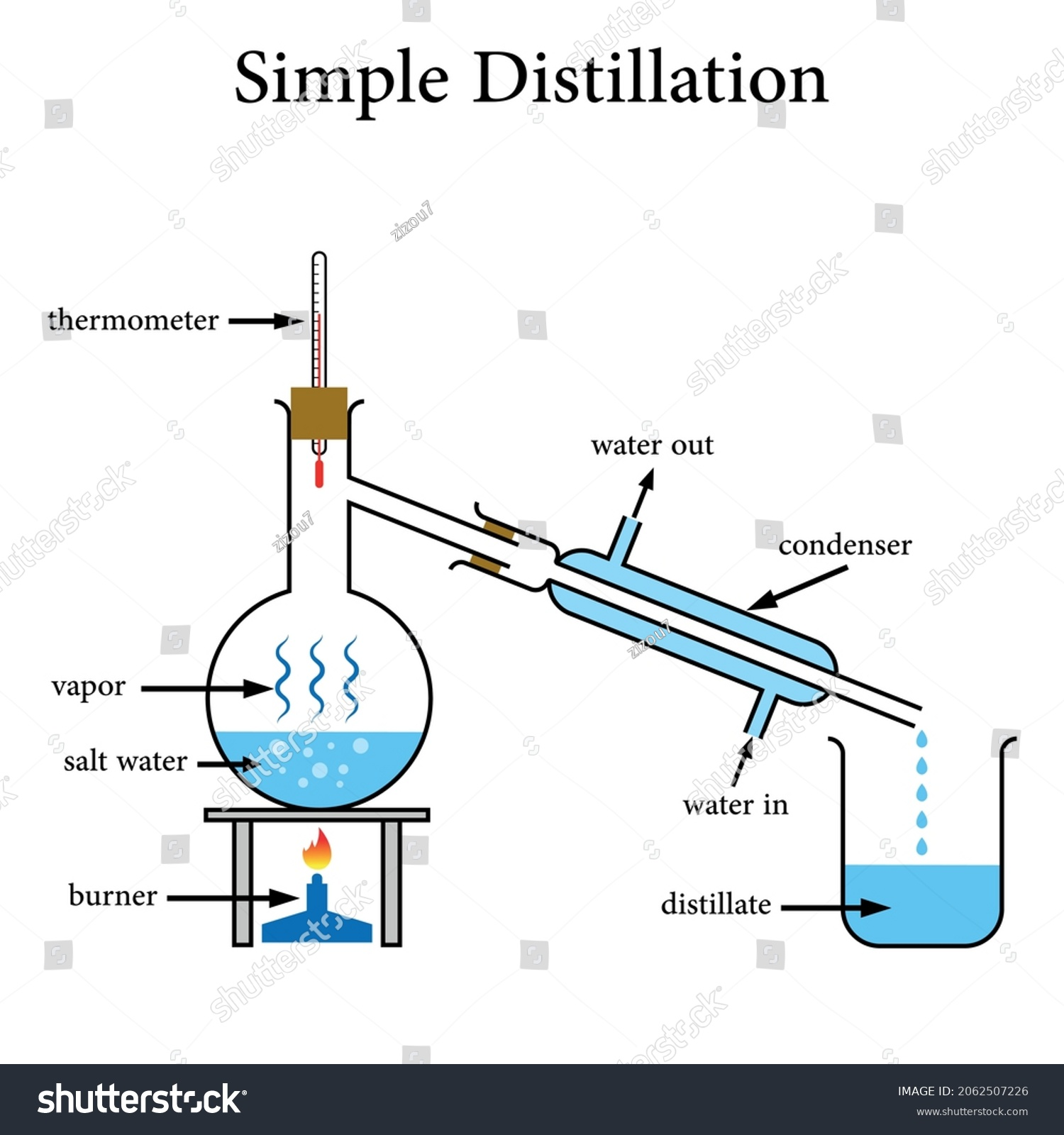
Simple Distillation Method
Used to purify liquids by heating and cooling (separates liquid from a solution)
Heat solution- part with lowest boiling point will evaporate first
Vapour rises to top of flask and travels down condenser where is cools, condenses and is collected in a beaker
Rest of solution remains in flask
Only very useful for separating mixtures made of liquids with very different boiling points
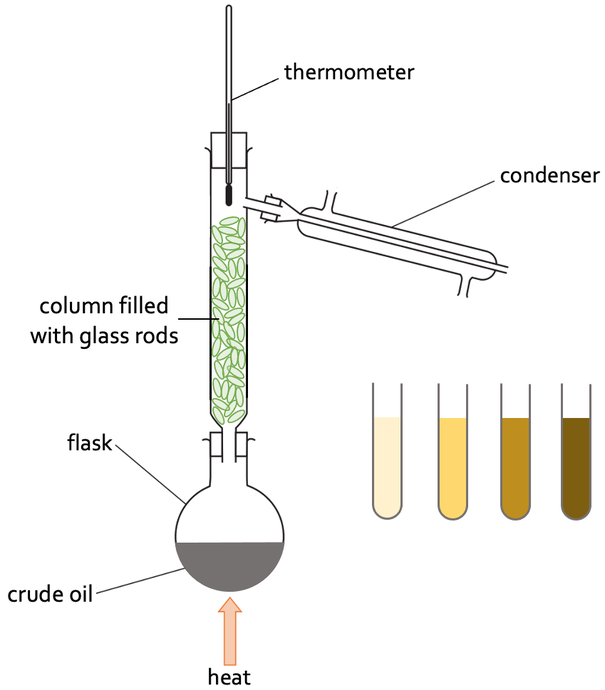
Fractional Distillation Method
Used to separate mixtures made of liquids with close boiling points
Put mixture in flask and attach fractionating column on top and heat
Liquids will evaporate at different temperatures due to varying boiling points
Lowest boiling point liquid evaporates first. When thermometer reads the liquid’s boiling point, it has reached top of column
Other liquids may start to evaporate, but the column is cooler towards top so they won’t fully make it up while the other liquid is at the top
when all of first liquid is collected, increase temperature until the next liquid reaches the top and repeat until all liquids are separated
The glass rods n the fractionating column provide more surface area for the liquids to condense on
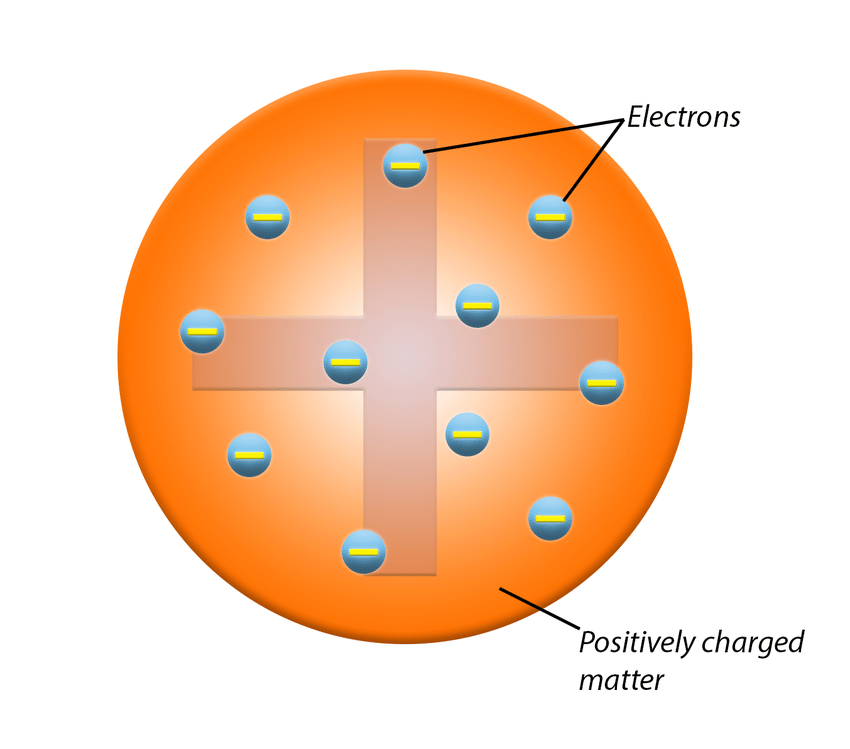
Plum Pudding Model
1897 by JJ Thompson
Ball of positive charge with negative electrons inside
Alpha Scattering Experiment + Atomic/Nuclear Model
1909 by Ernest Rutherford and student Marsden
Fired positive alpha particles (helium nucleus) at thin gold sheet
Expected for particles to pass straight through or slight deflection- plum pudding model showed positive charge as spread out in atom
More deflected than expected and some were deflected backwards.
This proved there was a small concentrated positive nucleus with most of the mass while electrons surrounded it. Shows most of atom is empty space
When nucleus hit gold sheet, atoms deflected
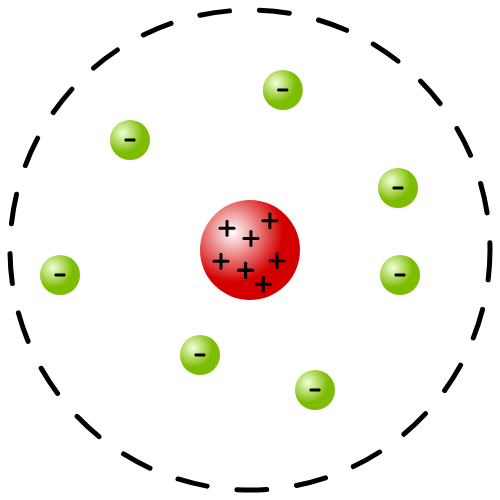
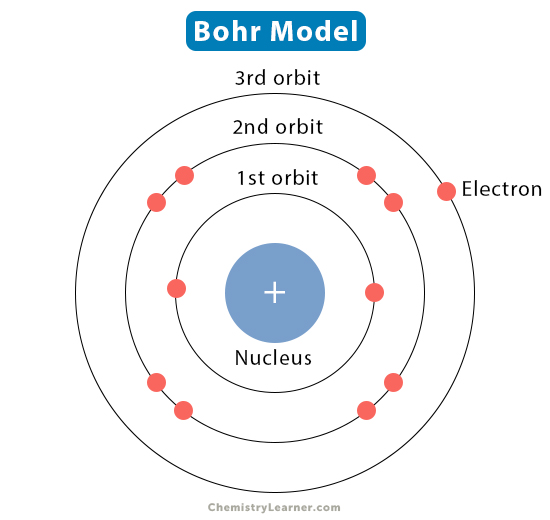
Improved Nuclear Model
1913- Niels Bohr suggested electrons orbit nucleus in fixed shells in fixed distance from nucleus
Order of Particles’ Discovery
Earliest → Latest
Electrons
Protons
Neutrons
Structure of Atom/Electron Shells
Closest electron shells filled first
1st electron shell: 2 electrons
2nd electron shell: 8 electrons
3rd electron shell: 8 electrons
etc.
Electronic configurations shown as 2:8:8
Electronic Structure of Carbon
2:4
Carbon has Atomic number of 6, so has 6 protons
Number of protons = Number of electrons
So 6 electrons
Only 2 electrons on first shell, and rest are on 2nd shell
Electronic Structure of Chlorine
2:8:7
Chlorine has Atomic number of 17, so has 17 protons
Number of protons = Number of electrons
So 17 electrons
Only 2 electrons on first shell and 8 on second shell
Leaves 7 more electrons which all fit on 3rd shell
Mendeleev’s Table of Elements
1869- Dmitri Mendeleev
50 known elements into Table of Elements
Organised based on atomic mass but also in order based on properties
Included gaps for undiscovered elements with gaps in correct spaces dependant on their properties
Modern Periodic Table
Metals on left, Non-metals on right
Elements in order of increasing atomic number
Periods- rows
Groups- vertical columns organised based on properties e.g. alkali metals, noble gases and halogens
Groups also determine number of electrons in outer shell e.g. group 1 elements have 1 electron in outer shell (group 0 elements have full outer shells)
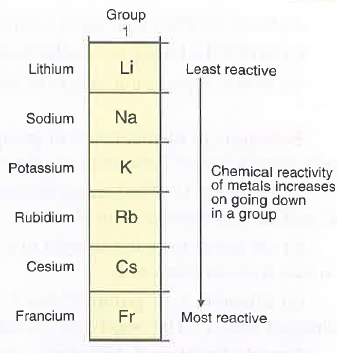
Alkali Metals
Group 1 of Periodic Table
Reactive; Soft; Low density; Metals
Have 1 electron in outer shell
Form +1 ions (lose 1 electron to make full outer shell)
Trends as you go down Group 1:
Reactivity increases
Lower melting + boiling points
Higher atomic mass
When reacted with water, produce hydrogen
When reacted with chlorine, produce salt
When reacted with oxygen, produce metal oxide
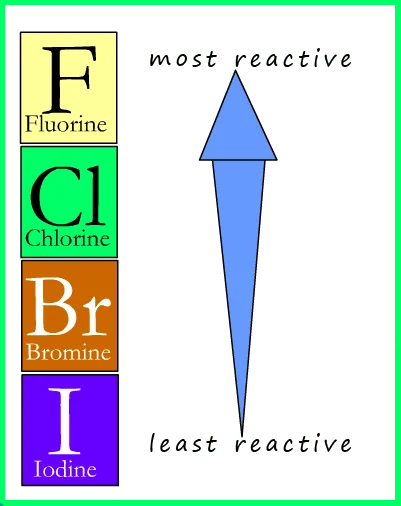
Halogens
Group 7 of Periodic Table
Reactive; Non-metal; Diatomic
Have 7 electrons in outer shell
Form -1 ions (gain 1 electron to make full outer shell)
Trends as you go down Group 7:
Reactivity decreases
Higher melting + boiling points
Higher atomic mass
More reactive halogens displace less reactive ones (e.g. fluorine would displace chlorine and any of the other halogens in a reaction)
Properties of each Halogen
Fluorine:
Yellow gas; Very reactive; Poisonous
Chlorine:
Dense; Green Gas; Fairly reactive; Poisonous
Bromine:
Red-brown volatile liquid; Dense; Poisonous
Iodine:
Dark grey crystalline solid OR purple vapour; Less reactive; Solid at room temperature; Poisonous
Noble Gases
Group 0 of Periodic Table (far-right)
Colourless; Inert; Non-metals; Monoatomic Gases
Have full outer shells
No need to lose/gain electrons to be stable (inert)
Trend as you go down Group 0:
Boiling point increases
Boiling point increases due to more electrons which leads to stronger intermolecular forces
