UConn MCB 2210 Exam 2
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
How do proteins get localized to different places in the cell?
The Nucleus
The eukaryotic cell is characterized by
distinct functional compartments
Each cell compartment is referred to as an
organelle
Each organelle is bounded by at least
one membrane
There are multiple destinations for proteins in eukaryotes
• There are cytosolic proteins
• There are extracellular (secreted) proteins
• There are plasma membrane proteins
• There are proteins in the membrane(s) of each of the organelles
• There are proteins inside (in the lumen) of each organelle
The majority of proteins are synthesized on
cytosolic ribosomes
Production of cytosolic proteins
• mRNA exits nucleus (more later)
•Binds free ribosome in cytosol
•Protein is translated and folds (maybe with help of chaperones and/ or chaperonins)
gated transport of proteins
nuclear - whole folded protein is selectively moved through aqueous pores into a topologically similar compartment
Transmembrane transport of proteins
folded or unfolded protein moves via nonaqueous transport complexes into a topologically distinct compartment.
vesicular transport of proteins
protein is transported in the lumen or membrane of small vesicles that fuse with the target compartment; topology conserved.
posttranslationally
Transport after the whole protein has been made
cotranslationally
transport simultaneously with protein synthesis
Gated transport is always
posttranslational
Transmembrane transport can be
post- or co translational.
Vesicular transport only occurs after
transmembrane transport
Postranslational transmembrane transport usually involves cytosolic
chaperones, which keep the protein unfolded in the cytoplasm
No label means
"cytosolic protein" If a protein is made in the cytoplasm and has no targeting signal, it will stay in the cytoplasm (default localization).
•Non-cytosolic proteins contain
short sequences of amino acids which act as the targeting signal. Specific labels exist for nucleus, mitochondria, endoplasmic reticulum, etc
Nuclear envelope
double membrane surrounding the nucleus
Outer membrane of the nuclear envelope is continuous with the
Rough ER
Nuclear pore complex controls
entry and exit
Nuclear lamina-
meshwork of intermediate filaments (lamins) under the nuclear envelope
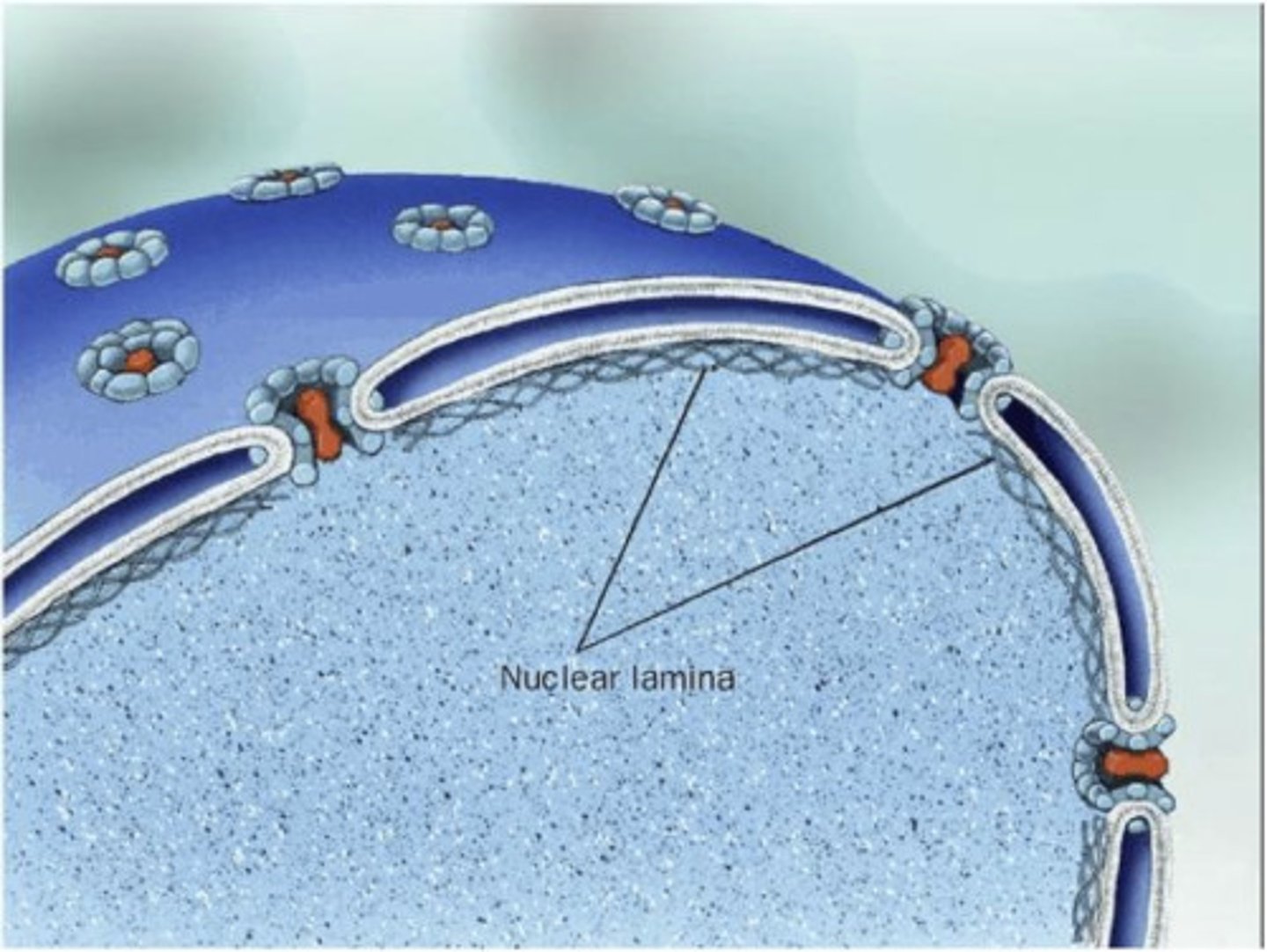
• Nucleoplasm
fluid phase of nucleus (like cytoplasm)
Chromatin-
DNA/protein complex
Nucleolus
DNA/protein complex housing the rRNA genes for production of ribosomal RNA. Ribosomal subunits assembly
Since protein synthesis occurs in the cytoplasm, all nuclear proteins are
imported
Nuclear proteins:
DNA polymerase, histones, lamins, etc
Small nuclear RNAs (snRNAs) are transported to the cytoplasm, assembled with proteins and then transported
back into the nucleus as snRNPs that regulate splicing.
The Nuclear Pore Complex controls
entry and exit
Ribosomal subunits are assembled in the nucleolus (in nucleus) and then transported
out into the cytoplasm
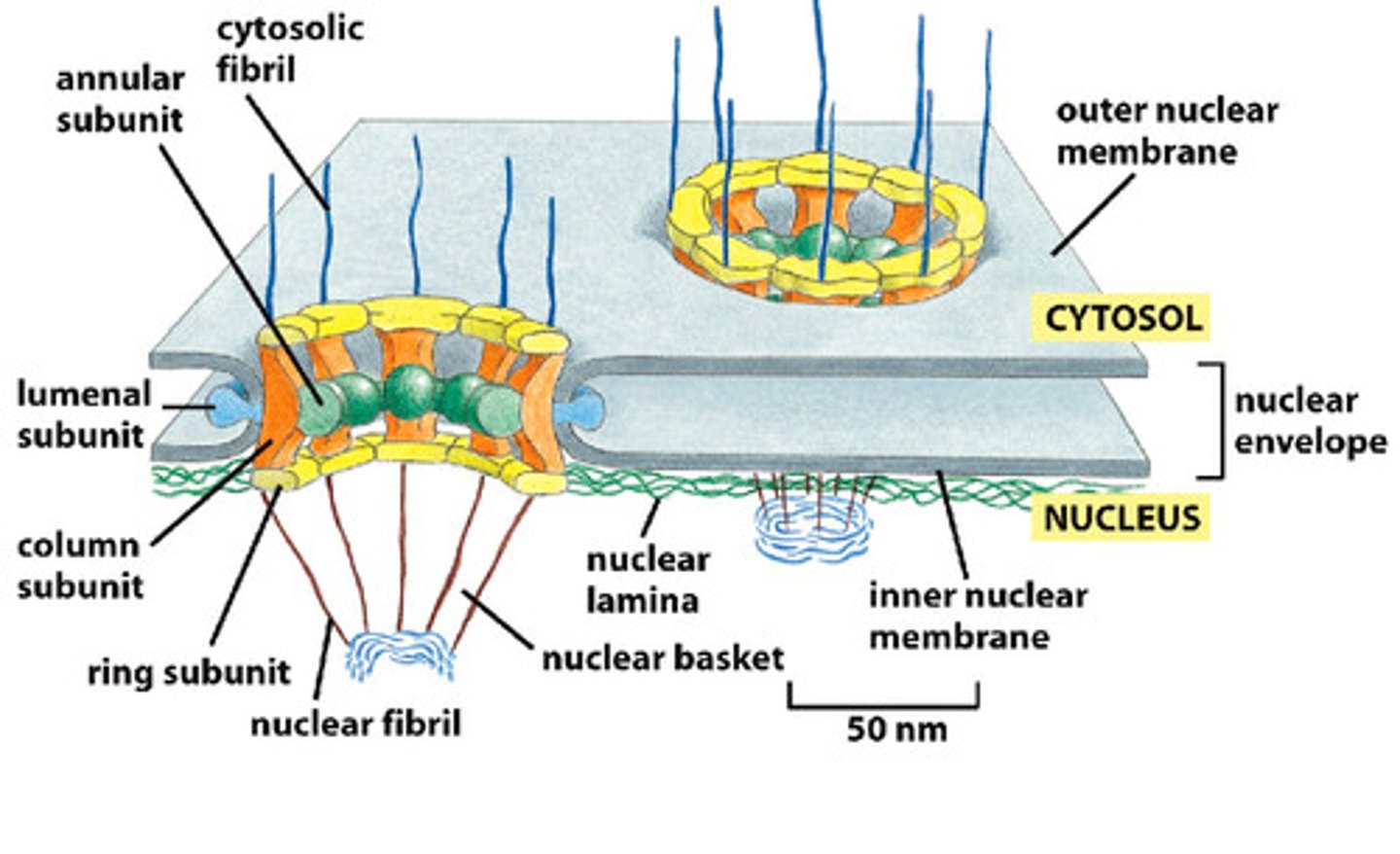
Nuclear Pore Complex
• A huge complex with a ring, basket and plug-like structure
• can see features by EM
• Made up of about 50 proteins
• 3000-4000 per nucleus in typical cell
• 30x the mass of a ribosome
• Can even trick cell to transport a whole ribosome through it!
Molecules smaller than
5000 MW (5kDa) move freely through the nuclear pore by passive diffusion
Larger molecules move more slowly by diffusion
Proteins and complexes that are too large to diffuse are actively transported by a process that is not completely understood
The Nuclear Localization Signal
The signal sequence for the nucleus that enables proteins to move through pores in the nuclear envelope.
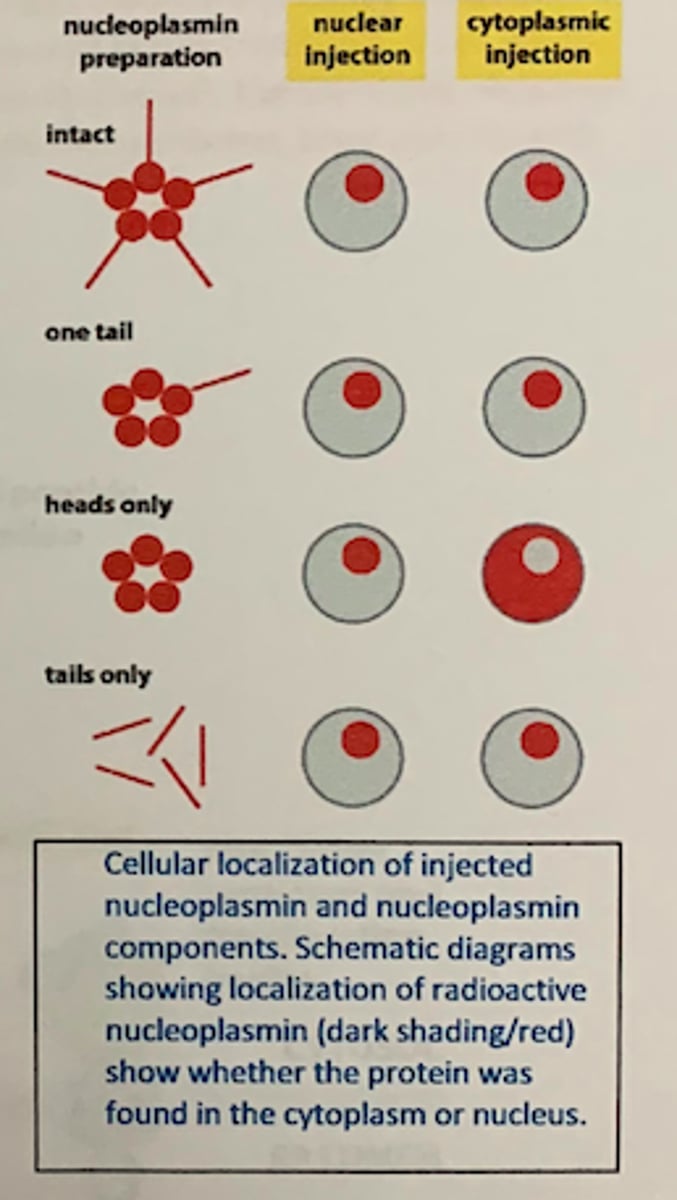
nucleoplasmin injection experiments
The whole protein can be taken up into nuclei • The tail is both necessary and sufficient for uptake (the head is neither necessary nor sufficient)
Proteins destined for the nucleus all have
a + charged group of 5-7 amino acids near the C-terminal end of the protein
Nuclear Localization Signals
• Proteins destined for the nucleus all have a + charged group of 5-7 amino acids near the C-terminal end of the protein
• The sequence is not the same for all nuclear proteins, but all are similar and + charged
• Transport is postranslational and proteins are already folded
• The + charged region must be on the surface of the folded protein so it can interact with transport proteins or pore
• This signal is necessary and sufficient to direct the uptake of any protein into the nucleus
• The signal is recognized by other proteins that regulate transport through the pore
• The signal is not removed upon entry - It gets reused after every cell division since the localization is lost during cell division
• Transport is through an aqueous pore, not directly across a membrane
Karyopherins
importins and exportins
- Nuclear import receptors (importins):
help proteins get into the nucleus
Nuclear export receptors (exportins):
help proteins get out of the nucleus
A small G-protein called Ran regulates both
nuclear import and export
RanGAP exclusively in
the cytoplasm
RanGEF exclusively in the
nucleus (bound to chromatin)
RanGEF
makes RanGTP (nucleus)
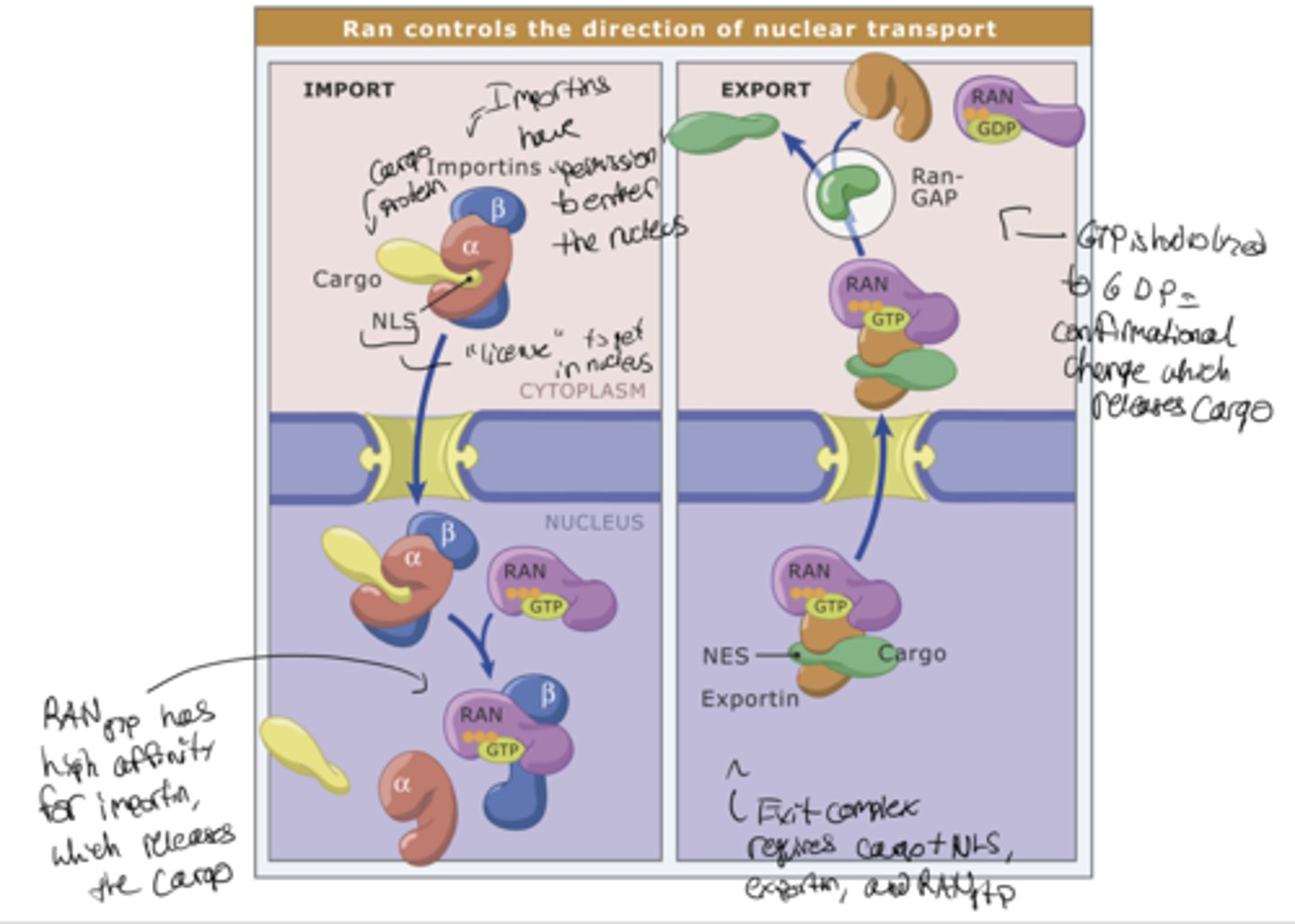
RanGAP
makes RanGDP (cytoplasm)
lots of Ran GTP in
nucleus
Lots of Ran GDP in
cytoplasm
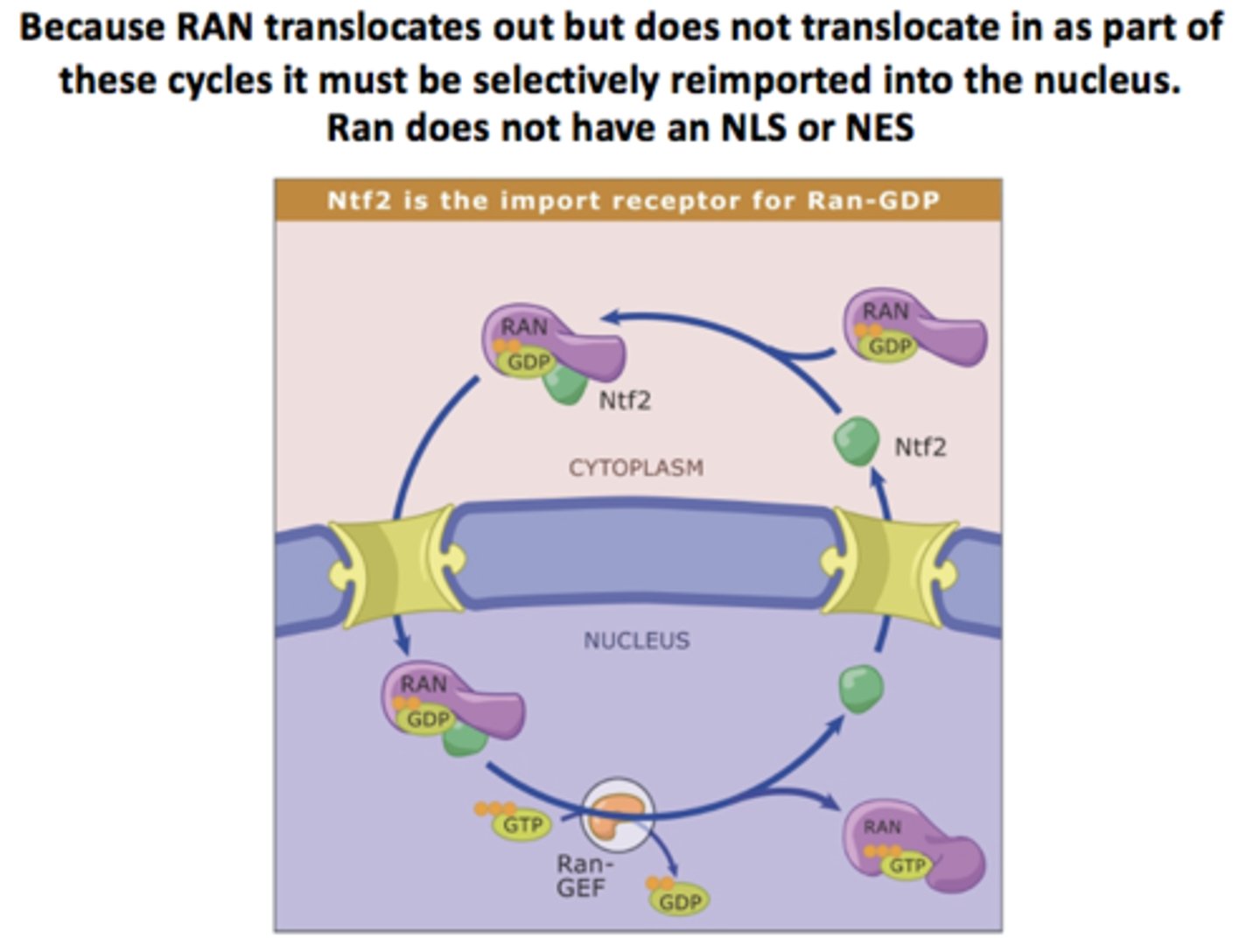
NTF2 (nuclear transport factor 2)
The nuclear import of Ran-GDP is mediated what separate transport receptor?
Transport of proteins across the nuclear envelope can be regulated
• A protein may have a targeting signal but not move through the pore.
- It may be bound to some other structure and immobilized
- The signal may be in the wrong conformation to be recognized (buried in the protein?)
- The signal may be concealed by another protein
• An upstream signal will cause a conformational change and allow nuclear import
Control of transcription factor import during T-cell activation
• Turn on response - NF-AT in cytoplasm of resting T-cell is phosphorylated. - Signals cause Ca++ to rise (what signals could cause this?) - Increased Ca++ allows calcineurin, a Ca++-dependent phosphatase, to bind to NF-AT. - Removal of PO4 from NF-AT exposes NLS. - NLS recognized by the import receptor. - Transported to nucleus, transcription turned on.
• Shut-off - Ca++ decreases; calcineurin releases NF-AT - NF-AT phosphorylated, hides NLS, NES exposed - Recognized by export receptor, exported. - Transcription turned off
Proteins destined for the cytoplasm have no
targeting sequences- this is the default pathway.
Larger proteins destined for the nucleus have an
NLS and are taken up fully-folded via nuclear pores (gated transport).
Proteins targeted for the Endoplasmic Reticulum also have
signal sequences, and are translocated across the ER membrane into a topologically distinct compartment (transmembrane transport).
ER Functions
• Site of protein synthesis for the endomembrane system.
- The secretory and endocytic pathways
- ER, Golgi, lysosomes, endosomes, secretory vesicles
- Plasma membrane
- Transmembrane and GPI-linked proteins
- Soluble proteins in the lumen of the endomembrane system
• Site of lipid synthesis
• Ca++ storage
• Enzyme storage in some cell types
-Detoxifying enzymes in liver cells
The ER is an extensive, dynamic structure
• Network (reticulum) of membrane tubules and sheets that stretches from the nucleus to the outer periphery of the cell.
• Constantly changing shape, extending and retracting
• During cell division, the entire network breaks down into vesicles that partition into daughter cells
RER ribosomes are bound to the membrane because
of the nascent chains (new proteins) they are making
• The proteins are being co-translationally inserted into the membrane (not post-translationally)
The ER signal sequence
a stretch of hydrophobic amino acids. - N-terminal signal sequence for soluble and some membrane proteins (a.k.a signal peptide, start transfer sequence). - Internal sequences for many membrane proteins.
• Leads to co-translational translocation across ER membrane.
Signal Recognition Particle
• SRP is found free in the cytoplasm.
• Discovered as a factor that allowed attachment of pure ribosomes to ER membranes.
• SRP is a ribonucleoprotein particle (RNP)=RNA+6 proteins.
• Binds to both the signal sequence and the ribosome.
• Binding of the SRP to the nascent peptide chain halts translation when the peptide is 70-100 AA long (long enough to protrude from the ribosomes).
One way to make single pass transmembrane proteins:
encode a stop transfer sequence
This applies to so-called Type I single-pass proteins
Another way to make a single pass transmembrane protein:
the internal start transfer sequence
• An internal start transfer sequence a.k.a. signal anchor sequence
Sequence is both the signal and the transmembrane anchor.
Type II
A Type II transmembrane protein has positively charged amino acid residues at the N-terminal end of its signal anchor sequence
N side on outside for
Type II
N side on inside for
Type III
How would you know if co- and post-translationally translocated proteins used the same translocation channel?
competition experiments
Most proteins imported into the ER become
glycosylated.
- Critical for quality control of folding in the ER
- Further processing of these sugars in Golgi for proper protein function
Oligosaccharyltransferase
is an integral membrane protein associated with the translocon complex.
sugars are added co-translocationally during import
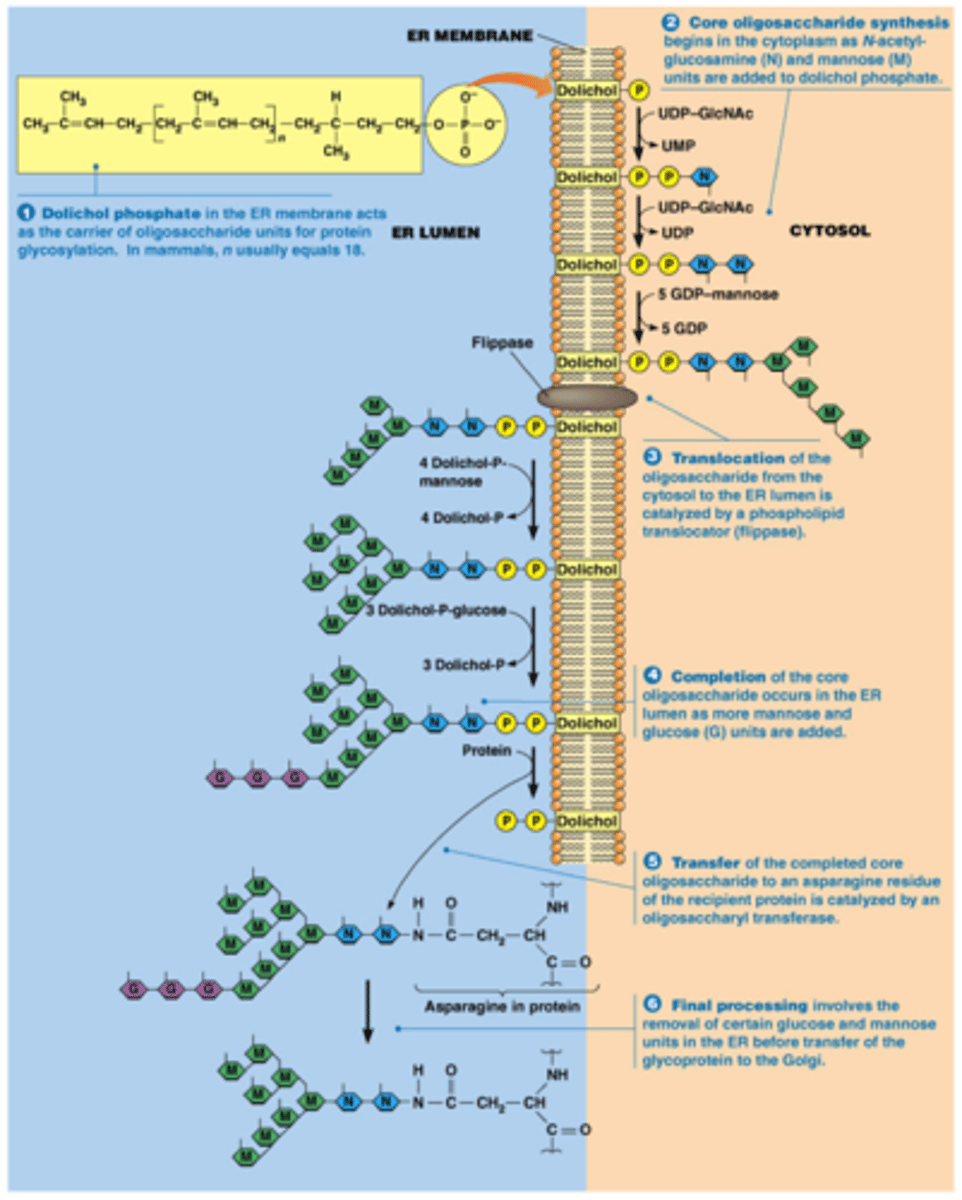
N-linked Glycosylation
the signal for glycosylation is the amino acid sequence Asn-X-Ser and addition is to a nitrogen in Asn.
This only happens in the lumen of the ER- there are no oligosaccarides of this sort on the cytoplasmic proteins
dolichol phosphate
Lipid anchored Proteins
• Some proteins are held in the membrane by lipid anchors
• GPI (glycosyl-phophatidyl-inositol) anchoring is done in the ER
• Begins as TM protein. An enzyme cleaves the protein and attaches it to the GPI anchor at the same time
• GPI-anchored membrane proteins face the lumen or ultimately the extracellular space if in plasma membrane • These proteins can be rapidly released from membranes by cleaving the anchor
Protein folding in the ER
• Chaperone proteins in the ER lumen aid in folding - Heat shock proteins (Hsc, Hsp)
- the family of proteins that either aid in protein folding or protect the cell from unfolding in response to stress (heat, chemical).
- Similar ER localized chaperones, such as BiP (Hsp70 family member), are constitutively expressed and bind unfolded or misfolded proteins in this compartment
• Protein disulfide isomerase (PDI)
- The lumens of endomembrane compartments, and the extracellular world, are harsher, more oxidizing environments than the cytoplasm.
- Disulfide bonds (S-S) that form between cysteines in oxidizing conditions can help to stabilize protein structure.
- Proteins have evolved differently depending upon where they are going to fold!
- PDI catalyzes the breaking and reforming of S-S bonds.
- The "shuffling" of disulfide bonds by PDI gives proteins a chance to be properly folded......
ER-associated protein degradation (ERAD).
- Misfolded proteins are recognized, modified and "dislocated" back across the ER membrane to the cytoplasm. Dislocation is a poorly understood mechanism, but may be the translocon with different accessory proteins acting backwards.
- During dislocation the proteins become "polyubiquitinated"; monomers of the small protein molecule ubiquitin are added in a chain by an E3-ubiquitin ligase.
- The cytoplasmic proteasome recognizes polyubiquitin and degrades the protein.
PDI
helps provide disulfide bonds
UGGT like hydrophobic stretches and
can therefore determine miss folded proteins
Mitochondria infolding called
cristae
Mitochondria inner most membrane
matrix
thylakoids
A flattened membrane sac inside the chloroplast, used to convert light energy to chemical energy.
- Inside of sacs: thylakoid space or lumen
- Outside of sacs: stroma or matrix
Targeting proteins to mitochondria
• Nuclear encoded mitochondrial proteins have a targeting signal
• This signal is an N-terminal amphipathic a-helix with (+) charged amino acids
- Necessary and sufficient for import
• Import is post-translational transmembrane transport
• The signal is bound by a receptor protein in the mitochondrial outer membrane
• Mitochondrial genome-encoded matrix proteins have
no signal; no need, made in matrix!
In Mitochondria unfolded proteins travel through
Tom and Tim transporters
Peroxisomes produce
H2O2 during the b-oxidation of very long chain fatty acids and other important oxidative reactions.
The Golgi has five distinct compartments
- Cis Golgi Network (CGN) - site of delivery from ER
- Cis Golgi - next region nearest the CGN
- Medial Golgi - the central region
- Trans Golgi - the distal region
- Trans Golgi Network (TGN) - site of sorting and exit of vesicles from Golgi network
Golgi Apparatus modifies
the oligosaccharides of proteins delivered from the RER
the er sends vesicles to the
cis golgi first
Different vesicle types have different coat proteins
- COPII- coats vesicles moving ER to Golgi
- COPI- coats vesicles moving Golgi to ER and from more trans Golgi to more cis Golgi
- Clathrin- coats vesicles moving from TGN to endosomes, lysosomes and from surface to endosomes, lysosomes.
A class of small G proteins regulates budding
These cytoplasmic G proteins have a lipid anchor that is exposed when GTP bound, or hidden when GDP bound, allowing them to reversibly associate with membranes. - GTP bound > anchor exposed > budding is initiated - GDP bound > anchor hidden > no budding, or removal of coat
Different types of coat proteins use a different G protein:
-COPII - Sar1
-COPI, Clathrin - Arf
GEF gives
ATP
Some soluble proteins seem to be carried along non-specifically by
"bulk flow"
transmembrane cargo receptors interact directly with
coat proteins via their cytoplasmic domains.
Rab family of small G-proteins( involved in docking
• GDP bound - inactive, cytoplasmic
• GTP bound state - active; tightly associated with membranes
• RabGTP mediates tethering and docking of vesicles by binding to Rab effector proteins in target membranes
• Over 60 different Rab family members! The specificity of vesicular trafficking pathways is in part controlled by specific Rabs and effectors used for different routes.
• Activated by RabGEFs
Together, Rabs and SNARES control the specificity and directionality of vesicular delivery and fusion.
• V-SNARES in vesicle membrane are recognized by T-SNARES on the target membrane (provides directionality between compartments).
• Bring membranes into close apposition allowing fusion • Many unique T-SNARE and V-SNARE proteins exist and specific pairings mediate specific membrane fusion events
ER-Golgi vesicle trafficking
• Active Sar1 G-protein initiates membrane budding process
• Recruits cytoplasmic COPII coat proteins to drive budding
• Receptor bound proteins, membrane proteins and free proteins are recruited into the budding vesicle
• The vesicle buds
• The adaptor coat falls off
• Vesicle finds and docks its target membrane via Rab Gproteins, Rab effectors and SNARE proteins; SNARE complexes drive fusion
• COPII vesicle transport has thus contributed to the CGN
ER export signals can
interact directly or indirectly with coat proteins. Along with bulk flow, this moves proteins to the Golgi and beyond.
Proteins that are supposed to function in the ER are recycled back to the ER
- Protein Disulfide Isomerase; catalyzes Cys-Cys disulfide bonds
- Chaperones that aid in folding; Bip
- SRP receptor
An ER retrieval signal for soluble proteins is KDEL (Lys-Asp-Glu-Leu)
- The KDEL ER retention signal is at the C-terminus
- KDEL binds a KDEL receptor in the Golgi membrane
- KDEL receptor interacts with COPI coat proteins on cytoplasmic side
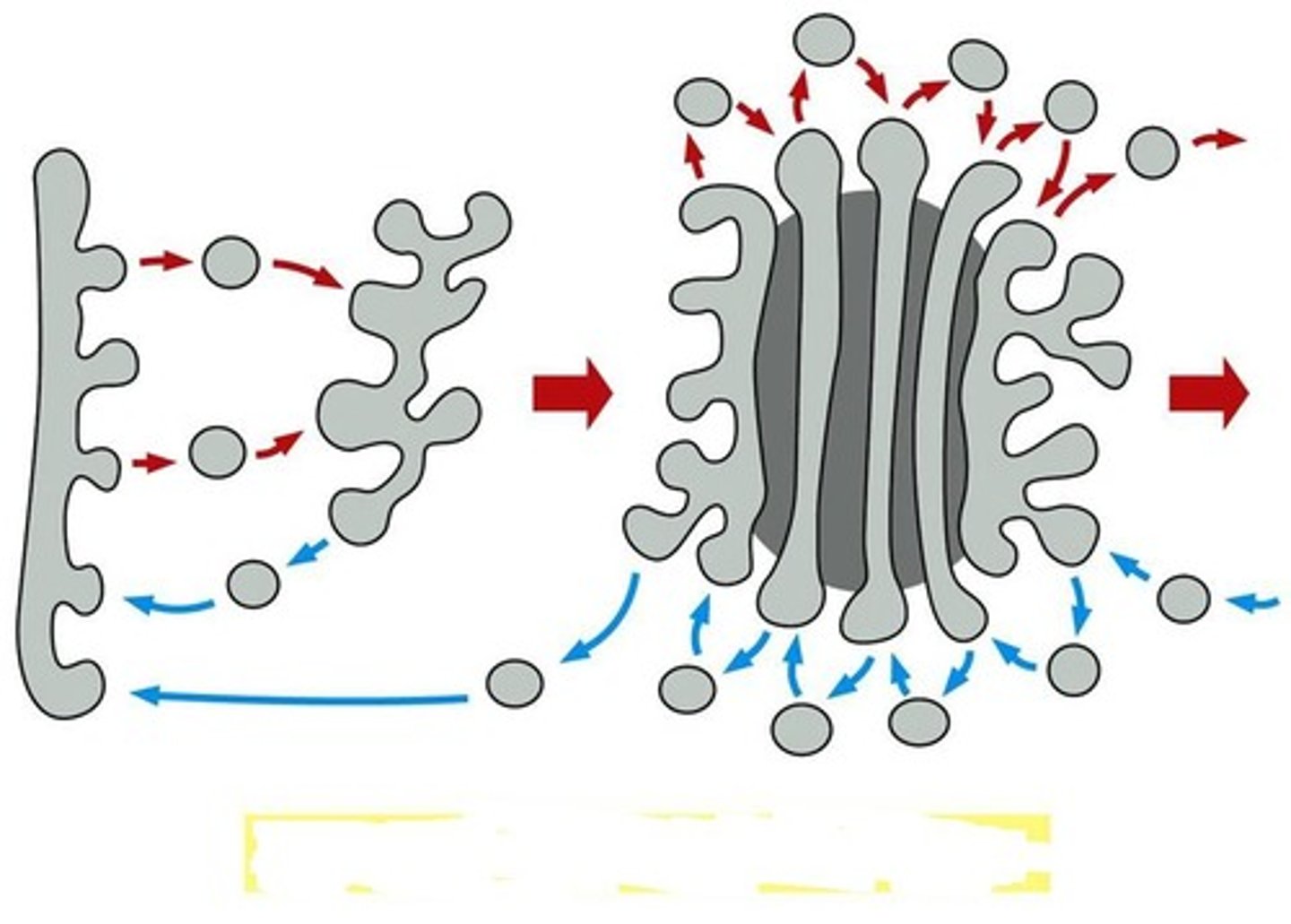
Vesicle Transport Model
Vesicles bud from one golgi compartment and fuse with the next to move cargo proteins and membrane forward from cis > medial > trans.
• The golgi compartments themselves are relatively static, with each maintaining their specific processing enzymes.
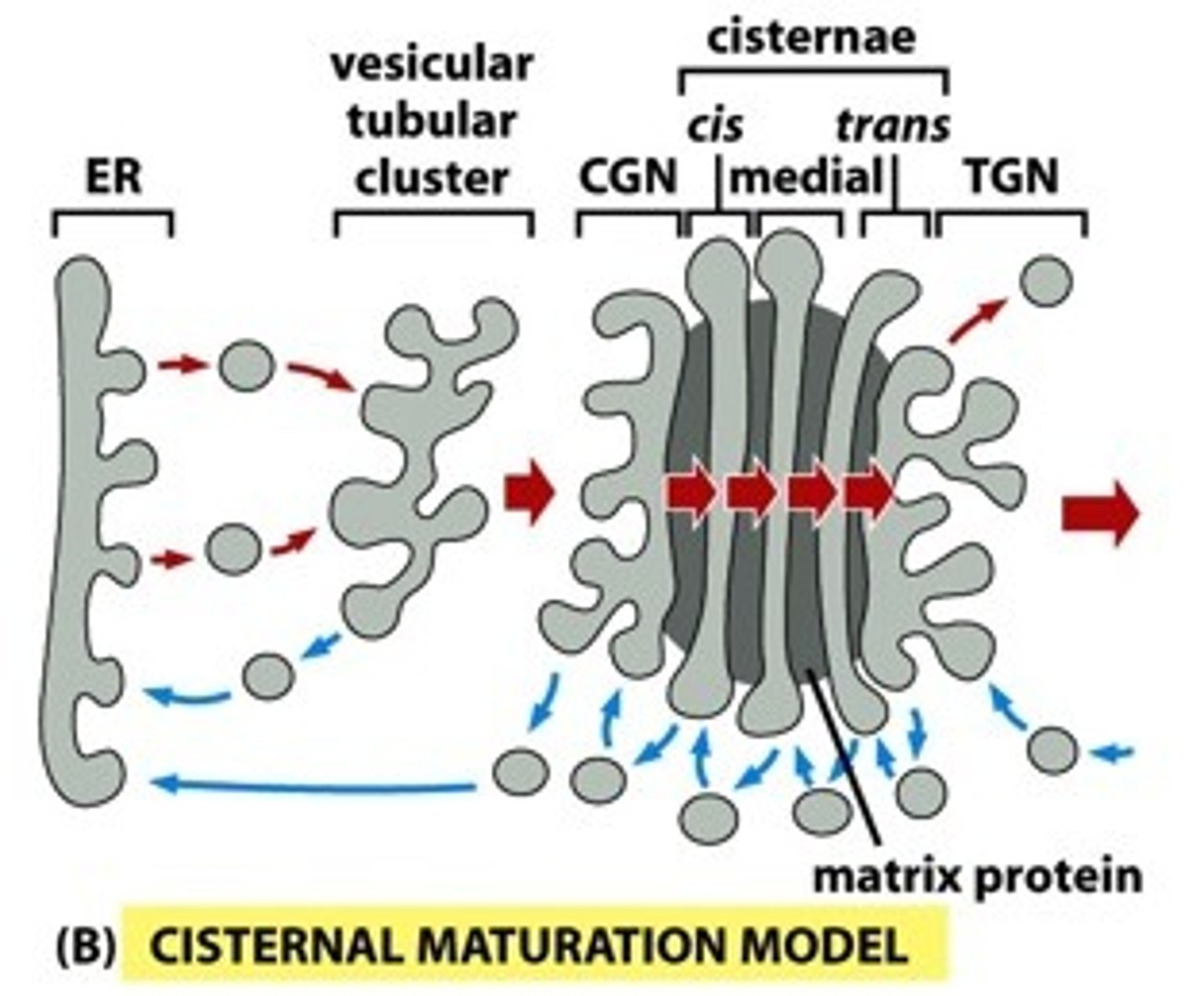
Cisternal Maturation Model
- A given golgi cisterna (compartment) is not fixed, but matures, both spatially and enzymatically.
• Cargo-containing COP-II vesicles from the ER fuse, generating a cisgolgi, and cargo is subject to cis-golgi enzymes in that cisterna.
• Meanwhile, another cis-golgi is forming behind the first, and the transgolgi at the other end of the network is diminishing, as it delivers its membrane bound cargo onward. In this way, the original ciscompartment is moving in the trans direction, becoming medial with respect to others, and ultimately will become TGN.
• At the same time, COP-I vesicles are mediating retrograde transport of enzymes from more trans- compartments to more cis- compartments. This means that a given compartment will be losing its cis- enzymes and gaining trans- enzymes; thus it "matures" from cis-, to medial-, to trans-golgi, as it is moving through the complex.
• The cargo remains in its original compartment as the enzyme composition of that compartment changes, and the compartment is displaced toward the trans region.
Lysosomes
cytoplasmic vesicles that take part in degradation of macromolecules.
•Internal environment is acidic
Clathrin
• The first coat protein discovered
• 3 heavy chains and 3 light chains form Clathrin triskelions
• Can self-assemble in test tube (in vitro) into polyhedral cage
• Arf regulated assembly presumably drives membrane budding
• Various Adapter Proteins (AP1, 2 etc) control cargo specificity
• I-cell disease
severe case in which none of the lysosomal enzymes are sorted correctly to lysosomes.
(Hurlers disease)-
a single type of enzyme is missing and accumulates certain types of macromolecules
inclusion bodies
persistent vesicles full of stuff that is normally broken down by lysosomes.