Topic 10: Polymers
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms

polymers are materials with structures made up of repeating molecular units, often based on carbon
each repeating unit is called a monomer
the small molecules or monomers are covalently bonded together to form long molecular chains
what are polymers
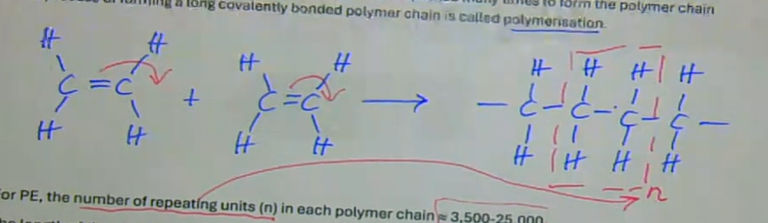
the double bond between the C atoms is ‘opened up’ and replaced by a single covalent bond allowing each C atom to bond to another molecule
this process is repeated many times to form the polymer chain — this process of forming a long covalently bonded polymer is called polymerisation
for PE, the number of repeating units (n) in each polymer chain is ~3500 to 25000
how are polymers formed
measure of the average number of repeating units/monomers in each polymer chain
calculated by:
DP = average molecular weight of chains in the polymer (g/mol)/molecular weight of the repeating unit (g/mol)
what is the degree of polymerisation (DP)
because the polymerisation reaction produces chains of variable length (variable DP)
why is the molecular weight always expressed as an average value
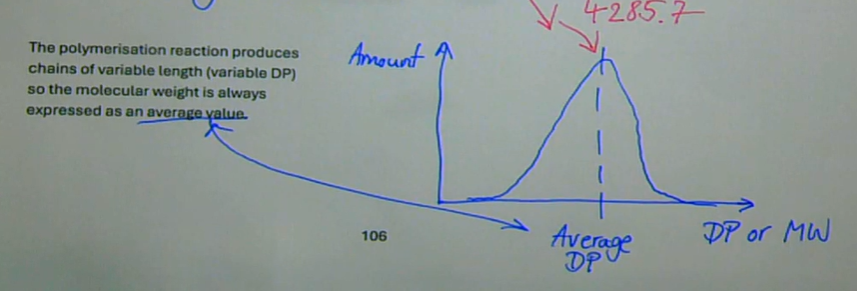
what does the DP graph look like
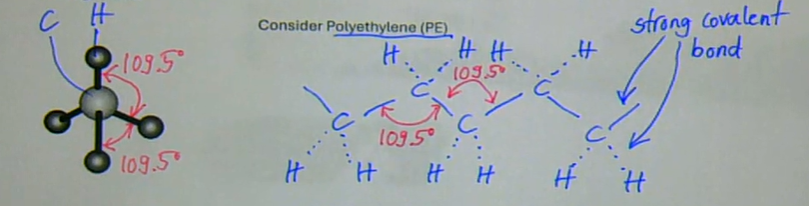
polymer molecules are not straight, as drawn in the previous section, but assume a zigzag configuration along the -C-C-C-C- backbone
this is because carbon is tetravalent → surrounded by four atoms in an equally spaced tetrahedral arrangement
describe the shape of a polymer molecule
the C-C bond has a fixed bond angle of 109.5 degrees, BUT rotation is possible about the C-C bond
the C-C bond is a covalent bond
this bond is strong and highly directional
describe the bond angle of C-C in terms of angle and strength
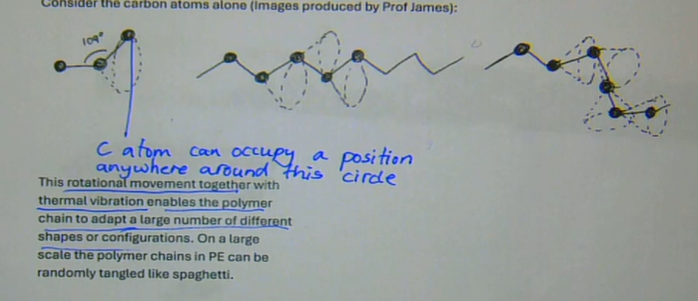
rotational movement together with thermal vibration enables the polymer chain to adapt a large number of different shapes or configurations
on a large scale the polymer chains in PE can be randomly tangled
how does rotational movement affect the shape of a polymer

what does the micro chain and bulk chain structure look like
strong covalent bonds within atoms in the chain
weaker secondary bonds between separate PE chains in the bulk polymer (Van der Waal’s forces)
tangling between chains
the degree of tangling increases with the length of the polymer chains and therefore with increasing DP
what are the three interactions that contribute to the mechanical strength of polymers
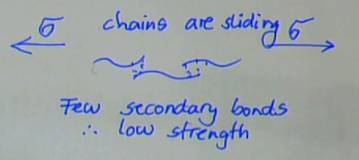
few secondary bonds
easy to break these and get chains to slide past each other under → low strength
describe short chain (low DP) polymers
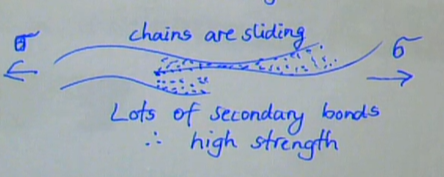
lots of secondary bonds
difficult to break all of these to get chains to slide past each other → high strength
also more strength from chain tangling
describe long chain (high DP) polymers
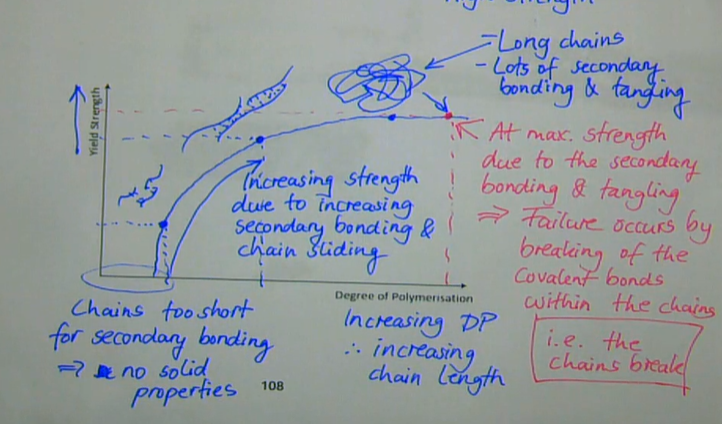
how does the graph of yield strength against degree of polymerisation look like
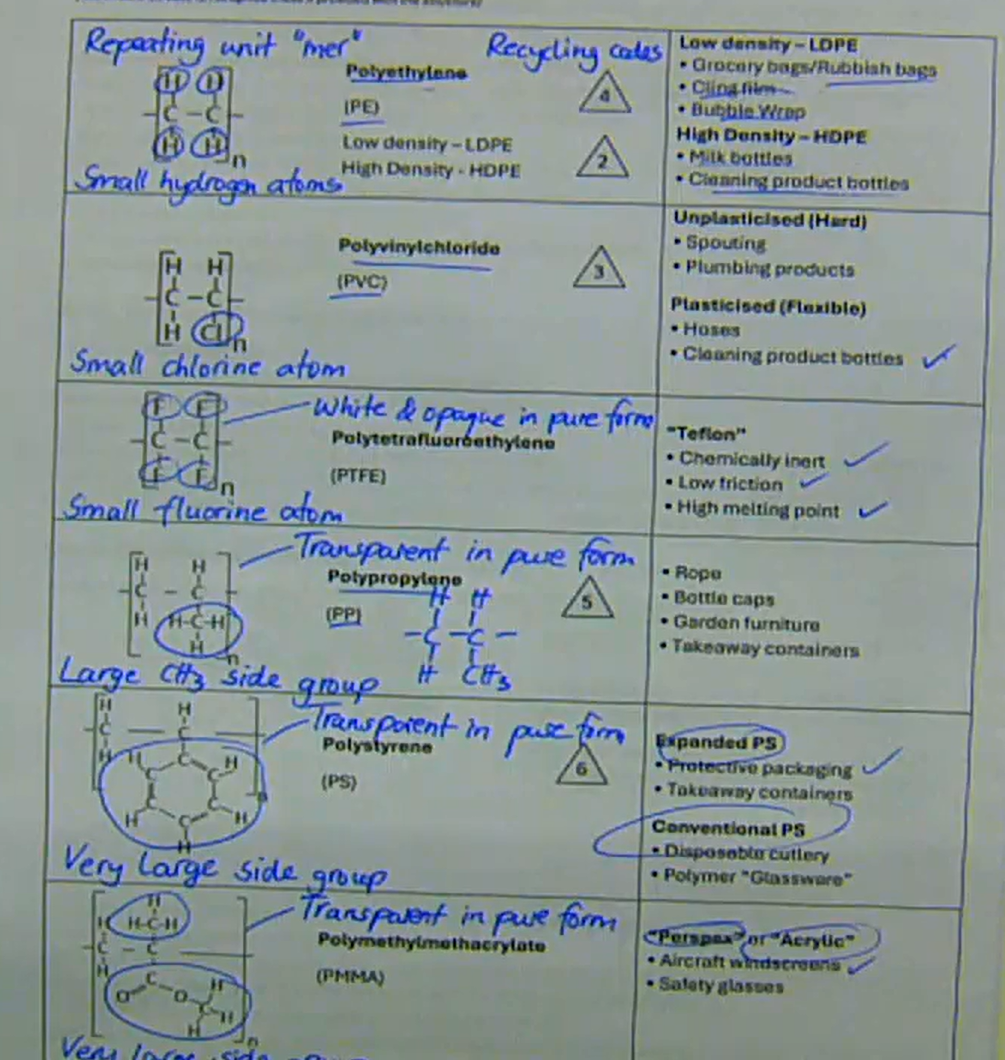
what are common polymers and their monomers
the same monomer repeating along the chain, e.g. -A-A-A-A-
what are homopolymers

two or more chemically different monomers, in different sequences
→ -A-B-A-B-A-B- → alternating copolymer
→ -A-A-A-B-B-B-A-A-A- → block copolymer
→ -A-BB-A-B-B-B-A- → random copolymer
→ -A/B-B-A/B-B-A-A-A/B-B-A-A/B-B → graft copolymer
what are copolymers
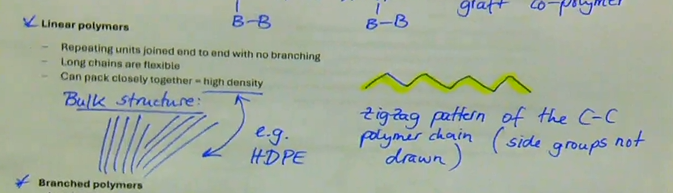
repeating units joined end to end with no branching
long chains are flexible
can pack closely together → high density
forms zigzag pattern of the C-C polymer chain
e.g. HDPE
what are linear atoms
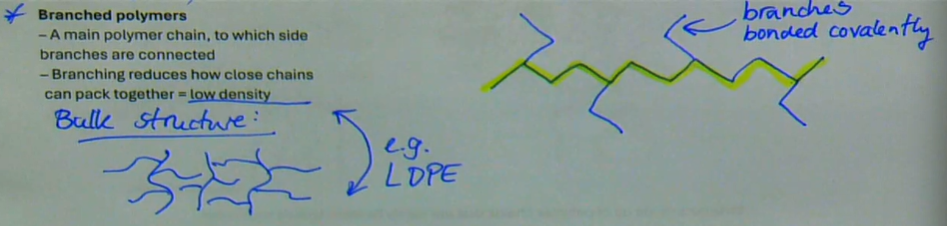
a main polymer chain, to which side branches are connected
branching reduces how close chains can pack together → low density
branches are bonded covalently
e.g. LDPE
what are branched polymers
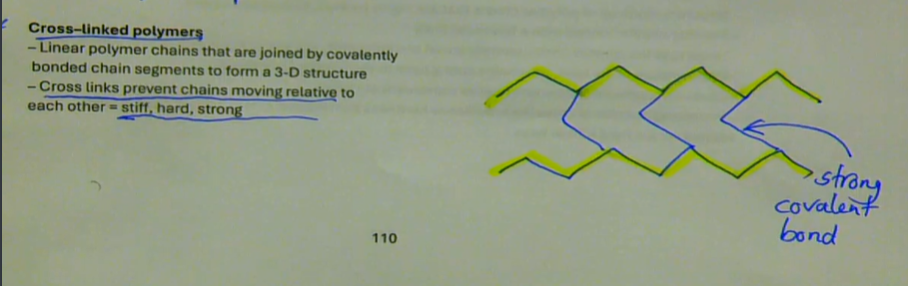
linear polymer chains that are joined by covalently bonded chain segments to form a 3-D structure
cross links prevents chains moving relative to each other → stiff, hard, strong
what are cross-linked polymers

linear/branched polymers with weak secondary bonds between chains
softens with increasing temperature
heat increases thermal vibration of polymer chains, which reduces secondary bonding and allows relative movement of the chains under applied stress
upon cooling, thermal vibration of the chains is reduced, allowing secondary bonding to increase, and polymer returns to its original properties
this process is reversible: polymer can be reheated and reshaped
allows thermoplastics to be recycled
what are thermoplastic polymers (thermoplsatics)

cross-linked polymers
strong covalently bonded cross links prevent ‘flow’ (permanent displacement) of polymer chains relative to each (do not soften with heating)
thermosets are harder and stronger than thermoplastics
thermosets cannot be melted, thus, cannot be recycled
at high temperatures they burn, degrade, and char
what are thermosetting polymers (thermosets)
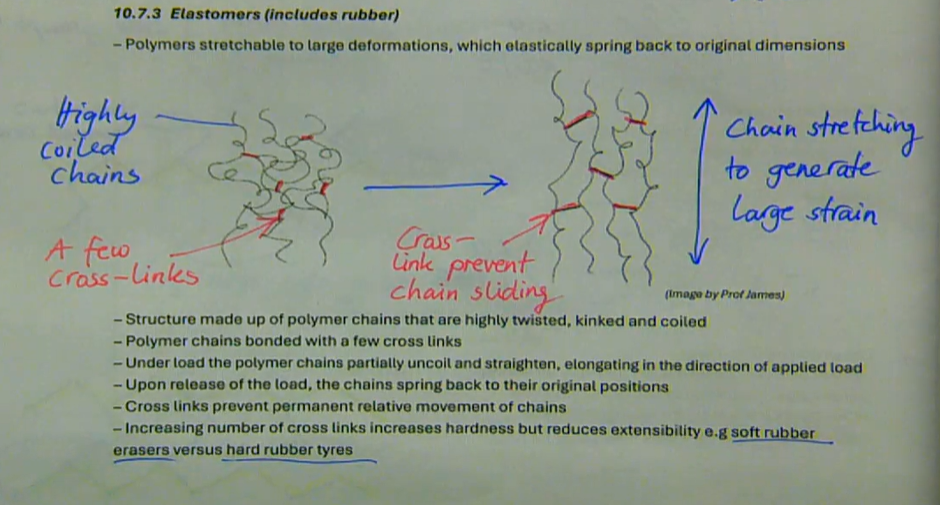
polymers stretchable to large deformations, which elastically spring back to original dimensions
structure made up of polymer chains that are highly twisted, kinked, and coiled
polymer chains bonded with a few cross links
under load the polymer chains partially uncoil and straighten, elongating in the direction of applied load
upon release of the load, the chains spring back to their original positions
cross links prevent permanent relative movement of chains
increasing number of cross links increases hardness but reduces extensibility, e.g. soft rubber erasers vs hard rubber tyres
what are elastomers

polymer chains mixed together with no long-range order
amorphous → transparent → low density
describe completely amorphous polymers
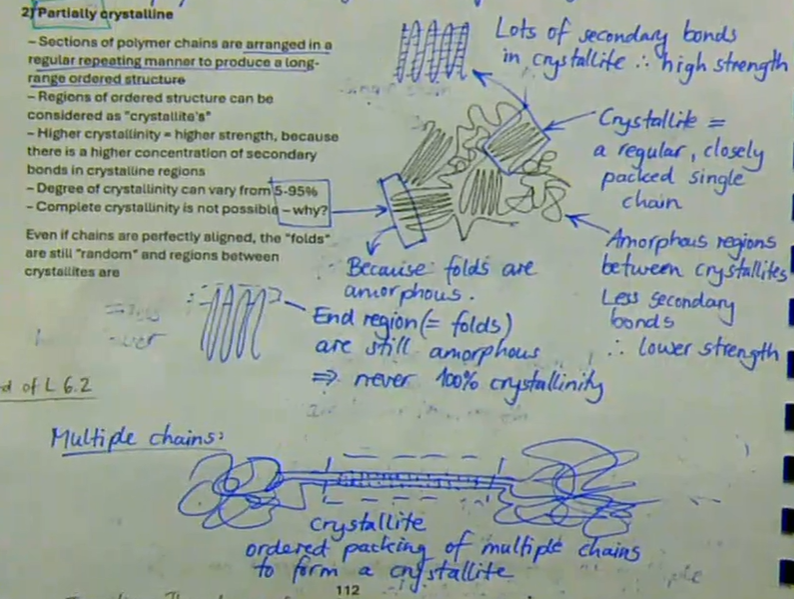
sections of polymer chains are arranged in a regular repeating manner to produce a long-range ordered structure
regions of ordered structure can be considered as ‘crystallites’
higher crystallinity → higher strength, because there is a higher concentration of secondary bonds in crystalline regions
degree of crystallinity can vary from 5 to 95%
complete crystallinity is not possibly → even if chains are perfectly aligned, the ‘folds’ are still ‘random’ and regions between crystallines are
describe partially crystalline polymers
rate of cooling: high rate of cooling from the liquid state → low crystallinity (i.e. amorphous)
→ because long tangled chains need time to move and align themselves in an ordered manner to make crystallites
side groups: large bulky side groups (e.g. PMMA) → low crystallinity (i.e. typically amorphous)
→ because bulky side groups prevent chains from forming regular ordered arrangementarrangement of side groups along the chain: for a fixed polymer composition, the atoms and side groups attached to the main carbon chain can be arranged in different orientations
what are the factors that affect the degree of crystallinity

atactic form (e.g. PP)
→ random arrangement of side groups on either side groups on either side of the chain
the polymer is amorphous → because irregular position of side groups prevents chain packed in an ordered structure
mechanical properties are poor → because of low concentration of secondary bonds between irregularly packed chains
describe the atactic form
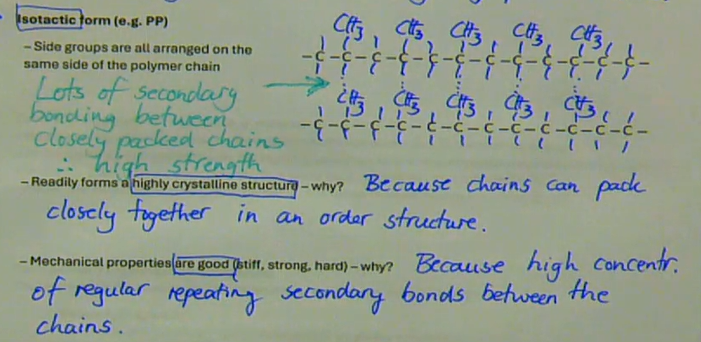
side groups are all arranged on the same side of the polymer chain → lots of secondary bonding between closely packed chains → therefore high strength
readily forms a highly crystalline structure → because chains can pack closely together in an order structure
mechanical properties are good (stiff, strong, hard) → because high concentration of regular repeating secondary bonds between the chains
describe the isotactic form (e.g. PP)
when a thermoplastic polymer is cooked from a flowable liquid state, it may form a completely non-crystalline (amorphous) structure, or it may form a partially or highly crystalline solid structure
what is the influence of temperature on thermoplastics
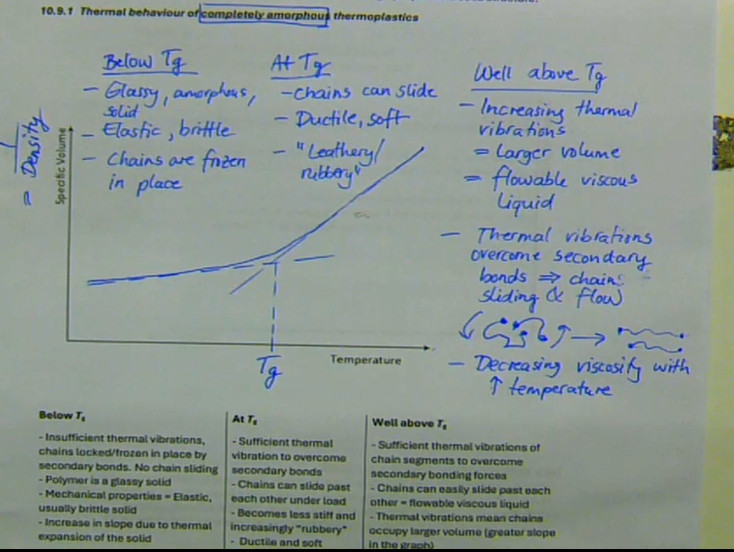
Below Tg:
→ Insufficient thermal vibrations
→ Chains locked/frozen in place by secondary bonds
→ No chain sliding
→ Polymer is a glassy solid
→ Mechanical properties = Elastic, usually brittle solid
→ Increase in slope due to thermal expansion of the solid
At Tg:
→ Sufficient thermal vibration to overcome secondary bonds
→ Chains can slide past each other under load
→ Becomes less stiff and increasingly “rubbery”
→ Ductile and soft
Well above Tg:
→ Sufficient thermal vibrations of chain segments to overcome secondary bonding forces
→ Chains can easily slide past each other = flowable viscous liquid occupy larger volume (greater slope in the graph)
what is the thermal behaviour of an amorphous thermoplastic
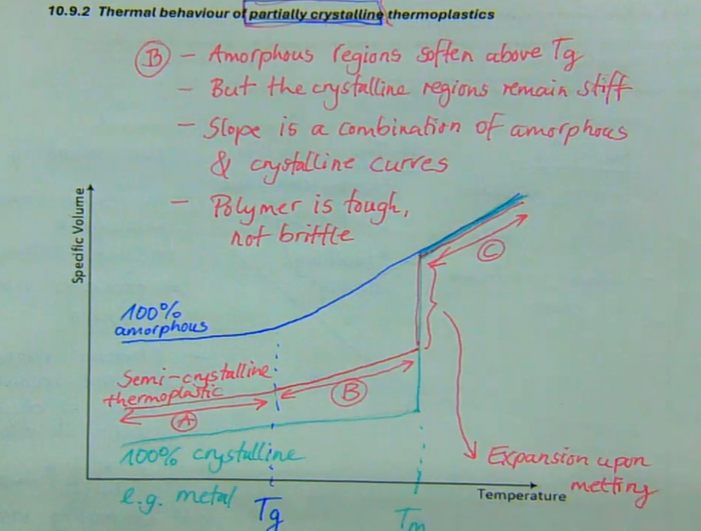
A → hard, brittle, solid
B → amorphous regions soften above Tg, but the crystalline regions remain stiff, slope is a combination of amorphous and crystalline curves, polymer is tough, not brittle
C → at Tmelt, the crystalline regions transform into amorphous material with low viscosity
what is the thermal behaviour of a partially crystalline thermoplastic

linear chains allow chain segments to line up easily
this means
→ high degree of crystallinity
→ crystalline regions have a lot of secondary bonding interactions between the chains → high-strength compared to amorphous regions with less secondary bonding
why does high density PE (HDPE) have high strength
LDPE has branches of PE grafted onto main PE chain
side branches prevent the polymer chain from packing closely together and generates low density structure
this also makes it difficult for crystallisation to occur
there are fewer secondary bonds between the branched chains compared to the linear HDPE chains and, therefore, it has lower strength
forms amorphous structure
what does low-density PE (LDPE) have low strength
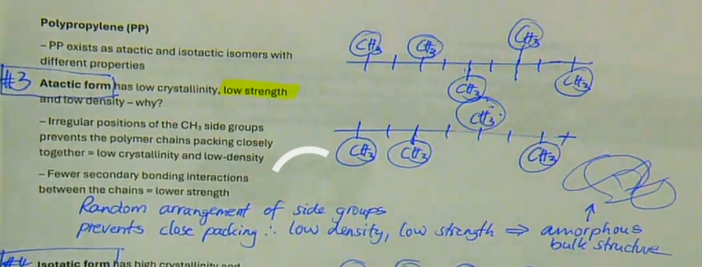
low crystallinity
low strength
low density
→ irregular positions of the CH3 side groups prevents the polymer chains packing closely together = low crystallinity and low-density
→ fewer secondary bonding interactions between the chains = lower strength
random arrangement of side groups prevents close packing therefore low density, low strength → amorphous bulk structure
describe the properties of atactic form
has high crystallinity and therefore high strength and density
→ regular repeated pattern of CH3 side groups allow chains to pack closely together = higher crystallinity and higher density
→ more secondary bonding interactions between the chains = higher strength
regular arrangement of side groups allow close packing therefore high density, high strength → semi-crystalline bulk structure
describe the properties of isotactic form

large bulky side groups prevent the polymer chains from aligning and packing closely together
therefore difficult to form crystals and so is fully amorphous
bulky side groups prevent close packing therefore low density, low strength → fully amorphous bulk structure
why is PMMA completely amorphous

if the dimensions of any crystalline regions in a polymer are greater than the wavelength of light, then internal reflection and refraction will occur, resulting in loss of optical transparency
highly amorphous polymers are generally optical transparent
transparency can also be changed by the addition of dyes or pigments, which can reduce transparency or make it opaque (as well as changes its colour)
because PMMA is fully amorphous:no order regions
→ no internal reflection and refraction, light travels through
→ transparent
semi-crystalline structure:if crystallite dimensions are greater than wavelength of light → opaque due to internal reflection and refraction
why is PMMA transparent
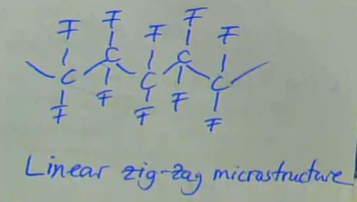
flourine is a very small, reactive atom
forms highly symmetrical chains which enables PTFE to be highly crystalline
→ lots of secondary bonding between the close packed chainshigh degree of secondary bonding = high strength, high density, and high melting point
strong C-F bonds = high chemical in inertness and ‘slipperiness’ (low coefficient of friction)
while it is a thermoplastic, it doesn’t melt normal thermoplastics and, therefore, must be sintered like a ceramic
linear zig-zag microstructure
F = small atom allows close chain packing therefore high density, high crystallinity (→ opaque)
high strength
describe PTFE — ‘Teflon’
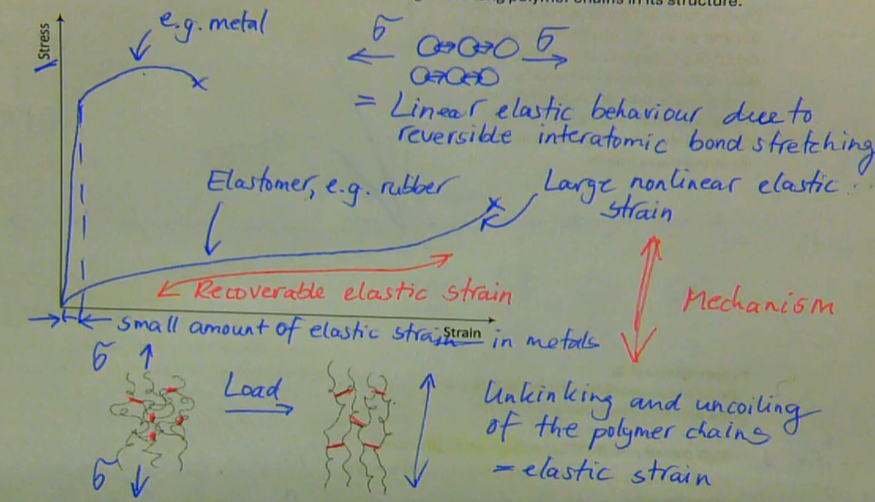
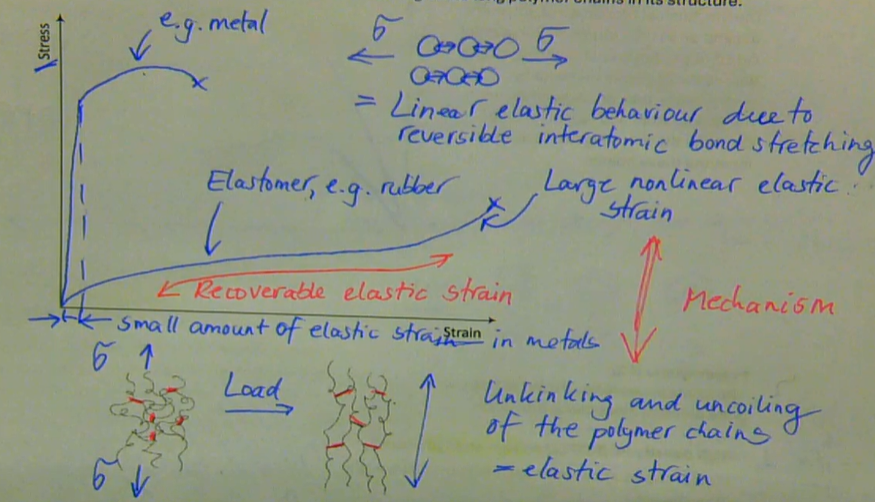
elastomeric polymers (e.g. rubbers) can exhibit very large non-linear tensile elastic strain (500 - 1000%) with full recovery, due to unkinking and rekinking of the long polymer chains in its structure
describe the mechanical response of elastomers
long, linear chains (i.e. high DP)
→ long chains allow high strain when stretchedrandom arrangement of polymer chains (i.e. must have an amorphous structure)
a) limited secondary bonding interactions between chains
b) amorphous structure allows for stretching by chain straighteningSufficient, but not too great, density of cross links between chains
→ cross links prevent irreversible chain sliding, but still allow chain stretchingchain segments must be in a state of constant thermal vibration
→ the elastomer must be above its Tg
→ thermal energy increases chain vibration which helps the chains unkink and slide past each other to generate elastic strain
what are the four structure requirements for large strain elastomeric behaviour
below Tg:
→ brittle solid, only elastic deformationabove Tg:
→ neck forms when yield stress is reached
→ neck becomes longer but not thinner going along stress-strain curve until fracture point is reached — called a travelling neck
→ because the curve gets higher, material becomes stronger due to orientation strengthening
describe the mechanical response of thermoplastics (viscoelasticity)
when load is applied:
→ amorphous chains align
→ becomes more crystalline
→ lots of secondary bonding
→ higher strengthamorphous material is drawn into aligned form in the neck → neck grows due to consumption of amorphous material from ends
why does the neck region in polymers become longer but not thinner
long tangled molecules are pulled into a ‘travelling neck’
chains become more aligned i.e. more crystalline
weaker in direction perpendicular to chains:
→ easy for a crack/tear to propagate along between the chainsstrong in direction along the chains:
→ hard for a crack/tear to grow across aligned chains
what is orientation strengthening

the deformation of thermoplastic materials can be primarily elastic, plastic (permanent) or a combination of both mechanisms
below Tg, thermoplastics deform primary by: elastic deformation
above Tg: thermoplastics deform primarily by: plastic deformation
deformation mechanisms:
1. stretching of bonds within the chain
→ undergoes elastic deformation
2. uncoiling and stretching of polymer chains
→ undergoes elastic deformation if the chains return to the original state upon unloading
→ undergoes plastic deformation if chains don’t return to original state at unload
3. chain sliding by breaking and reforming secondary bonding
→ undergoes plastic deformation
→ displays viscoelastic behaviour
describe the deformation mechanisms in thermoplastics
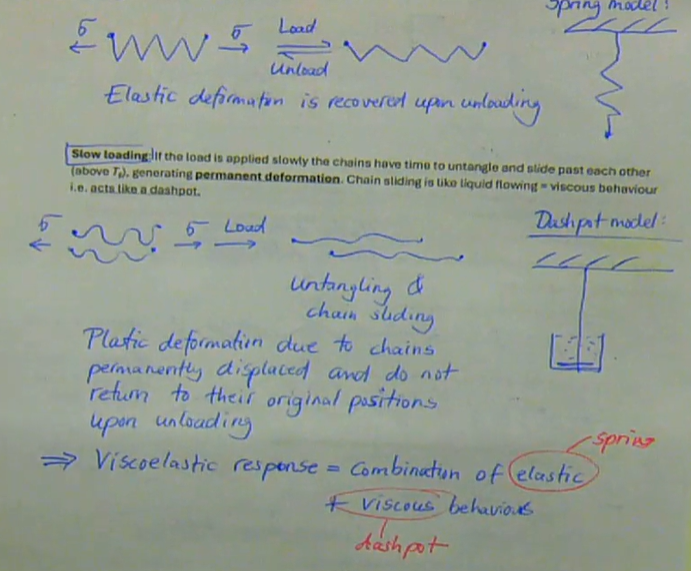
Rate of loading:
→ Fast loading: if the load on a polymer is applied very fast, the chains do not have time to untangle and slide past each other. Elastic deformation occurs by stretching of the bonds in the chain, i.e. acts like a spring. Elastic deformation is recovered upon loading.
→ Slow loading: If the load is applied slowly the chains have time to untangle and slide past each other (above Tg), generating permanent deformation. Chain sliding is liquid liquid flowing = viscous behaviour, i.e. acts like a dashpot. Plastic deformation due to chains permanently displaced and do not return to their original positions upon loading
this demonstrates the viscoelastic response of polymers → combination of elastic + viscous behaviour
what is the effect of loading conditions on polymer mechanical properties of Tg
under constant stress, polymers can exhibit
1. completely elastic → spring model
2. completely viscous → dashpot model
3. combination of both (viscoelatic) → Maxwell, Kelvin, or Voigt models
what are the viscoelastic models used to describe polymer behaviour
structure
temperature
rate of loading
time
what are the factors affecting viscoelastic behaviour
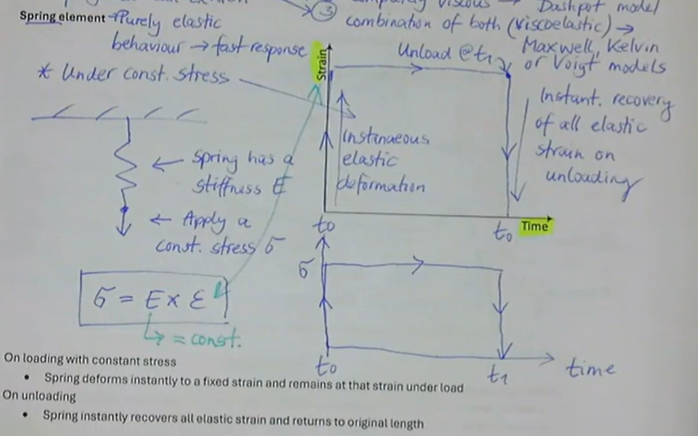
purely elastic behaviour → fast response
under constant stress:
stress = E (stiffness) x strain
what is the spring element
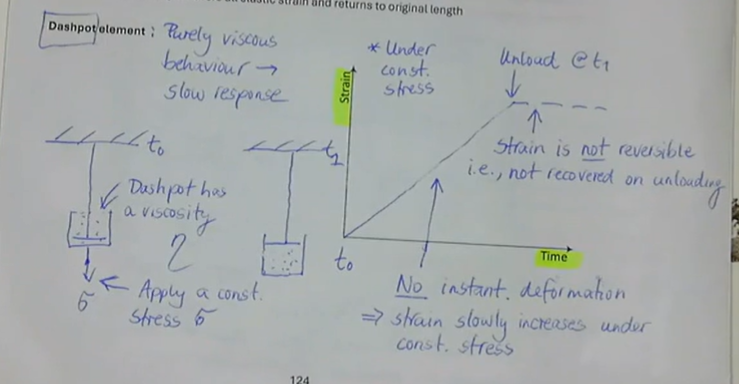
purely viscous behaviour → slow response
under constant stress:
no instant deformation → strain slowly increases
strain is not reversible, i.e., not recovered on unloading
what is the dashpot element
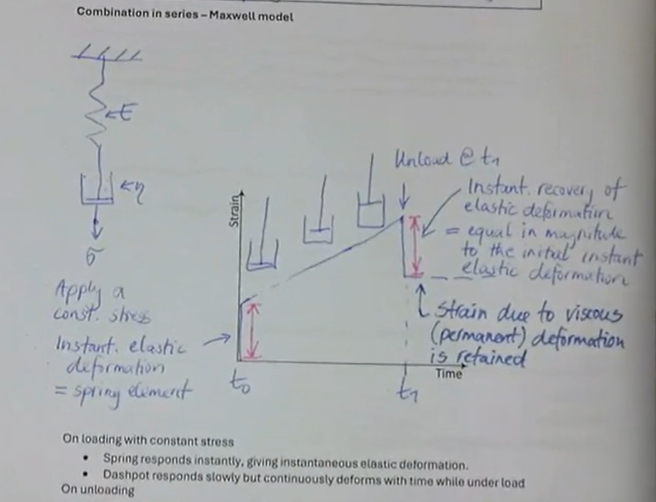
Combination in series → Maxwell model
under constant stress:
instantaneous elastic deformation = spring constant
after unloading there is an instantaneous recovery of elastic deformation → equal in magnitude to the initial elastic deformation
remaining strain is due to viscous (permanent) deformation is retained
what is the Maxwell model

Combination in parallel - Kelvin or Voigt model
under constant stress
spring and dashpot must deform at the same time, i.e., in parallel
responses up to the unloading point are a combination of elastic and viscous
upper limit of strain is due to max extension of spring
returns to zero strain upon unloading
what is the Kelvin/Voigt model

with most metals around room temperature we normally think of instantaneous elastic behaviour, i.e., a stress is applied and the metal deforms immediately to a small strain that allows us to calculate the metals elastic stiffness or modulus
E = stress/strain = constant
when <Tg: E = constant for a viscoelastic polymer → only elastic deformation
under these conditions of polymer chains, amorphous thermoplastics behave elastically just like ordinary metals
when > Tg: E is not constant
time-dependent stiffness/modulus is used
K(t) = constant stress/strain at 10 seconds
K(t) changes with temperature
what is polymer stiffness

at high temperature: viscous flow
chains slide past each other
modelled by dashpot
decreasing temperature: ‘rubbery’ response
decreasing temperature towards Tg (point of inflection): Transition region
‘leathery’ or semi-ductile
Kelvin/Voigt model
temperature less than Tg: Brittle, glassy, solid
chains frozen in place at low T
only elastic deformation by bond stretching
modelled by spring
stiffness increases with decreasing temperature
stiffness also increases with increasing rate of loading (deformation)
K(t)-Temperature graph