L42: Acid-Base Balance
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is an acid?
a substance that releases hydrogen ions (H⁺) when dissolved in water
pH level: < 7
taste: sour
What is a base?
a molecule that can accept hydrogen ions (H+) from an acid
pH level: > 7
taste: bitter
What is the pH value of stomach acid?
1
What is the pH of distilled water?
7
What is the pH of drain cleaner?
14
What is the normal pH for body fluids?
7.4
_____________ occurs when the body loses more H⁺ (acid) than it gains, causing the blood to become too basic (alkaline).
Alkalosis occurs when the body loses more H⁺ (acid) than it gains, causing the blood to become too basic (alkaline).
___________ occurs when the body gains more H+ (acid) than it looses, causing the blood to become too acidic
Acidosis occurs when the body gains more H+ (acid) than it looses, causing the blood to become too acidic
What is the kidneys’ role in acid-base balance?
They can adjust how much H⁺ (acid) or HCO₃⁻ (base) they excrete or reabsorb.
If the body gains acid → kidneys must increase base (HCO₃⁻) reabsorption or secretion
If the body gains base → kidneys must increase acid (H+) reabsorption or secretion
The kidneys eliminate or replenish hydrogen ions (H+) from the body by increasing OR decreasing the plasma _____________ concentration.
The kidneys eliminate or replenish hydrogen ions (H+) from the body by increasing OR decreasing the plasma bicarbonate (base) concentration.
How does buffering minimize changes in H+ concentration?
Buffers are usually negatively charged anions.
When H⁺ increases (more acidic), buffers bind the excess H⁺.
When H⁺ decreases (more alkaline), buffers release H⁺ back into the fluid.
A buffer is a molecule that stabilizes pH by reversibly binding H⁺ ions, preventing large changes in acidity.

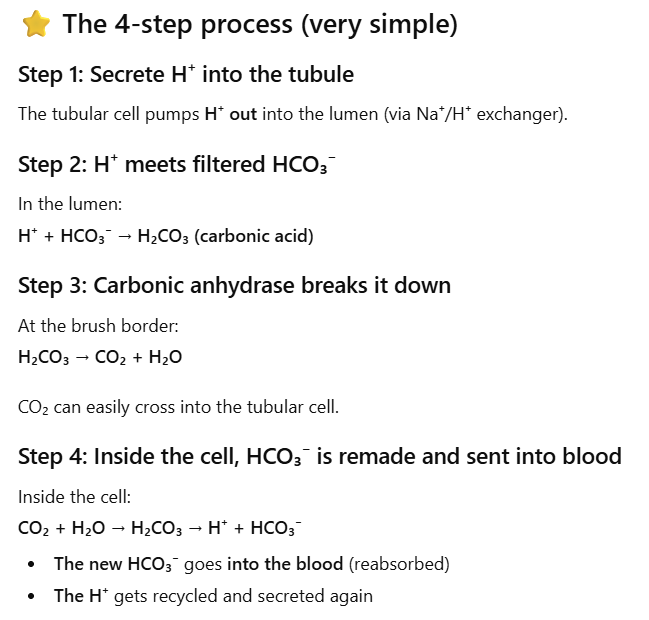
How do the kidneys reabsorb filtered bicarbonate (HCO₃⁻)?
HCO3- ions (base) are freely filtered into Bowman’s space (tubular lumen)
The kidney cannot directly reabsorb HCO₃⁻ from the lumen b/c the apical membrane does not have HCO₃⁻ transporters, so the body converts HCO₃⁻ into forms that can cross the membrane.
Tubular cells secrete H⁺ into the lumen (via Na⁺/H⁺ exchanger or H⁺ pumps). This H⁺ meets filtered HCO₃⁻ in the lumen.They combine to form: H⁺ + HCO₃⁻ → H₂CO₃ → H₂O + CO₂
H2O and CO2 are highly permeable through the plasma membranes.
The overall effect is reabsorption of the filtered HCO3- ions (base) into the blood
The body keeps the HCO3- (base) to buffer acids in the ECF (and plasma) → prevents acidosis
How do the kidneys respond to acidosis?
Make NEW Bicarbonate Using Urinary Buffers (Phosphate System)
Tubular cells produce new HCO₃⁻ (a base) in the nephron epithelial cell
This new HCO₃⁻ is added to the blood → raises pH (less acidic).
To get rid of excess acid, the kidney secretes H⁺ into the urine.
The H⁺ binds to urinary buffers other than bicarbonate (mainly phosphate).
The bound H⁺ is excreted in urine.
Make NEW Bicarbonate Using Glutamine (Ammonium System)
Kidney cells take up glutamine in the proximal tubule cell
Glutamine is broken down into:
HCO₃⁻ (goes to blood → raises pH)
NH₄⁺ (ammonium, an acid)
NH₄⁺ is secreted into the urine and excreted
How do the kidneys respond to alkalosis
decrease H+ ion (acid) secretion into the tubular fluid
decrease reabsorption of the filtered HCO3- (base)
decrease excretion of protonated phosphate (acid) and ammonium (acid)
increase renal HCO3- excretion in the urine
How do the kidneys maintain stable plasma pH?
Excrete bicarbonate (response to alkalosis)
Contribute new bicarbonate to blood (response to acidosis)
Excrete filtered phosphate
Excrete ammonium ions