topic 10 - ligand exchange and stability in d-block complexes
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
reactions with acid
most d-block metals react with acids to liberate H2 and form a metal salt
this involves the oxidation of the metal
the process is favourable with a negative delta G
can also be seen as the reduction of the metal ion is unfavourable - putting a negative standard electrode potential in the equation (delta G = -nFEo) leads to a positive delta G
delta G
= -nFEo
n = the number of electrons transferred per mole of reaction
F = Faraday’s constant: 96,485 C mol-1
a thermodynamically favourable process requires Eo > 0 and delta G < 0
standard electrode potentials
under standard conditions - standard states, 1atm, solutes at unit activity
always reported as reduction ie. forming the solid metal
values are relative to the reduction of hydrogen ions 2H+ + 2e- (eqm) H2
standard electrode potentials aren’t per mole so can’t be used in the same way as delta g when adding of subtracting equations
(when working with redox reactions its safer to work in delta g to calculate whether a reaction is favourable)
calculating K → delta G = ?
delta G = -(RT)lnK
R = 8.314 J K-1 mol-1
T = temperature in kelvin 298K
lnK = natural log (base e) of k
a thermodynamically favourable process requires K > 1
reaction with O2, S [S8], X2
d-block metals are reactive (usually at elevated temps) and readily produce binary compounds with O2, S [S8], X2
stereochemistry depends on the available oxidation states
disproportion reactions are also possible
Eo(Cu+/Cu) = 0.52V
Eo(Cu2+/Cu) = 0.34V
calculate E for: Cu2+ + e- → Cu+
delta G = -0.68F - (-0.52F) = -0.16F
Eo = -deltaG/nF = +0.16V
hydrolysis of ions in solution
dissolution of metal-salts e.g. MXn , in water produces complex ions ligated by H2O molecules
strongly polarising metal-aqua cations (eg Cr3+ (high charge density)water complexes) readily lose a proton to form an acidic solution and a metal-hydroxide species
as metal cation charge increases (above 4 ish) it becomes even more polarizing and two protons are lost to form an oxo (O2-) ligand
hydroxide and oxo ligands can lead to hydroxy or oxo bridged species with higher nucleophilicity (shown as (mu-OH)2)
stability of M2+ ions to oxidation
determining whether an ion will be oxidised or not cannot be done just by looking at electrode potentials - need to calculate delta G
why can Cu aqua complexes not displace all water ligands with ammonia
only 4 of the water ligands can be displaced as the gibbs free energy value for the 5th ammonia ligand is > 0 due to Jahn Teller effects
equilibrium stability constants
K1 represents the relative stability of the new complex
K2 = ? (Step wise stability constant)
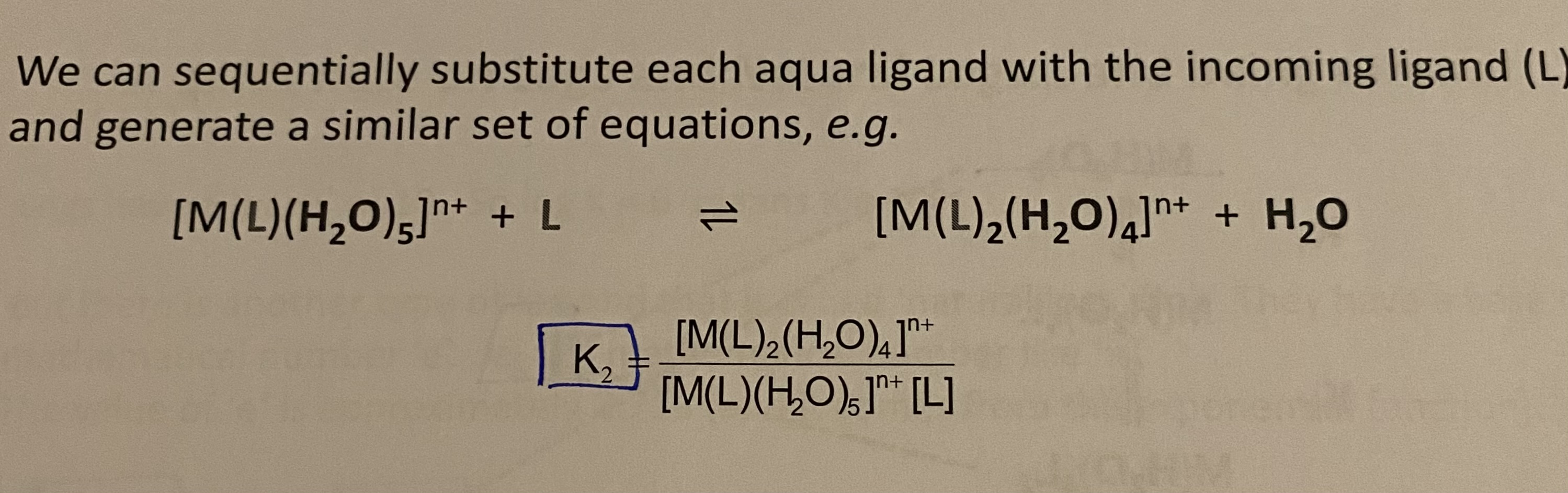
K6 = ? (Stepwise stability constant)
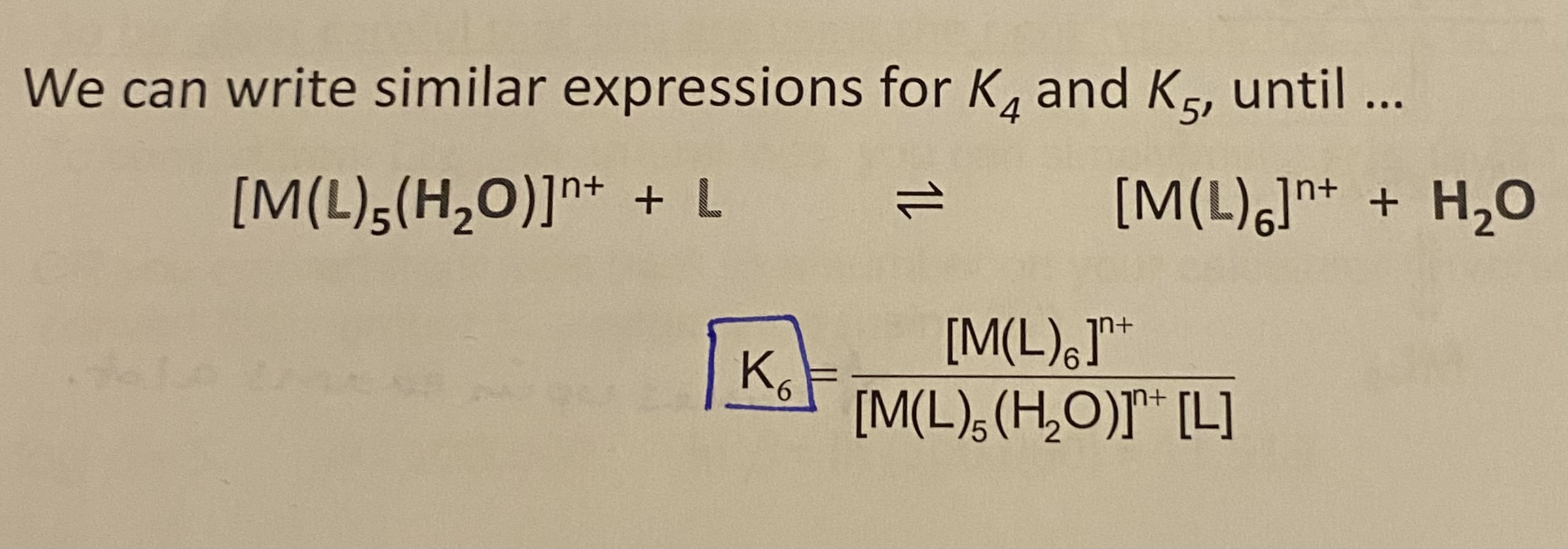
Overall stability/formation constant = ?
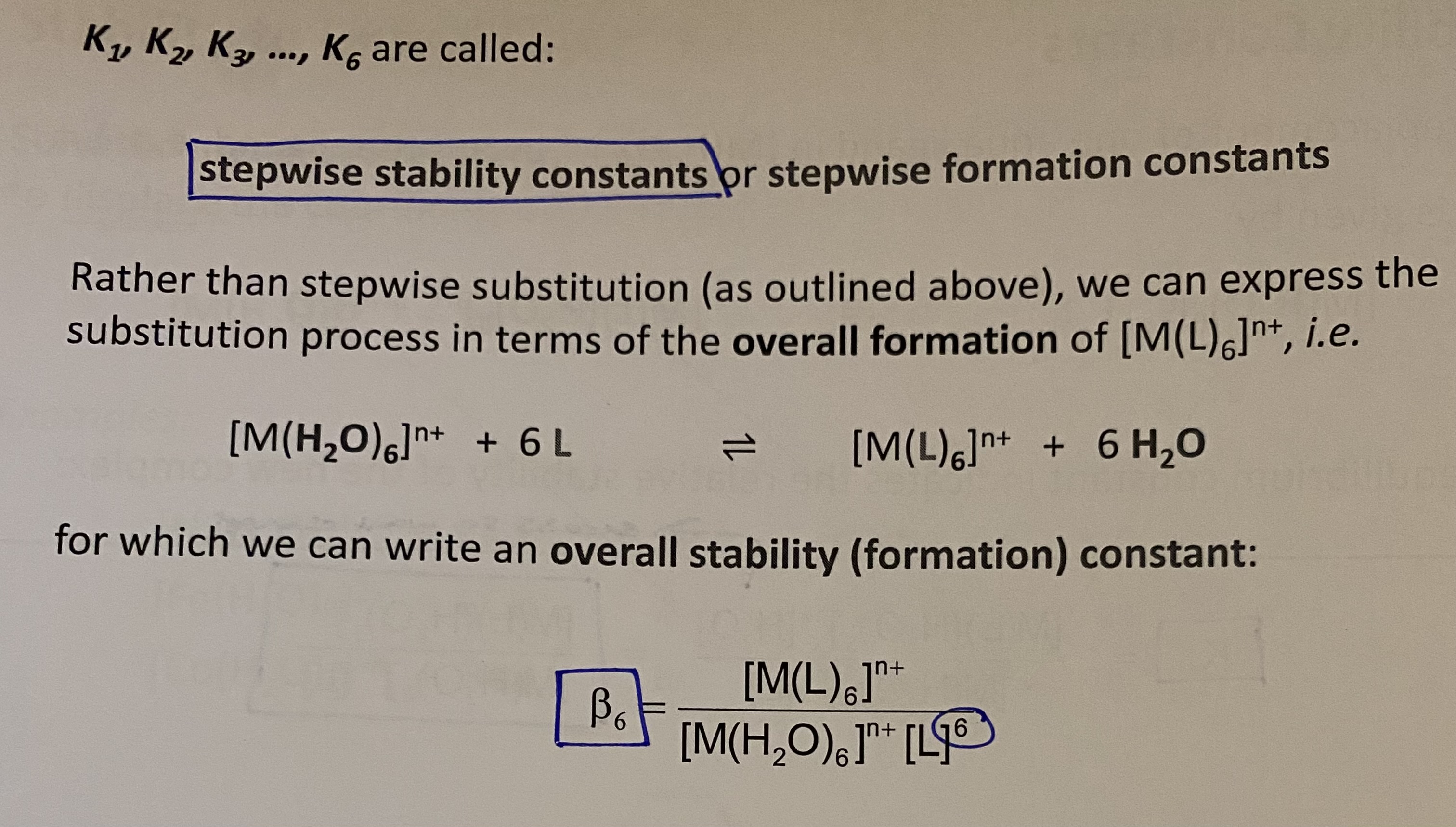
beta6 = ?
overall stability constant
K1 x K2 x K3 x K4 x K5 x K6
beta4 = ?
K1 x K2 x K3 x K4
log beta3 = ?
logK1 + logK2 + logK3
trend in stepwise stability constants
decrease as the constant number increases -based on probability
1st binding constant is higher bc whichever ligand is displaced the desired product will be formed but with the 2nd, 1/6 times you will end up back at the starting reactant rather than the desired product with two ligands substituted
K1 > K2 > K3 etc…
what causes deviation to the typical trend for stepwise stability constants?
changes in geometry (notably oct to tet)
for CuII (d9 Jahn Teller effects) 5th and 6th won’t go on
deltaG10 = ?
= -(RT)lnK1
the K in the original equation is equal to beta6
deltaGbeta2 0 = ?
= -(RT)lnK(beta2)
effects of CFSE on the kinetics of ligand exchange
to exchange a ligand an oct metal complex must go via a 5 or 7 coordinate intermediate
the crystal field splitting in 5 and 7 coordinate is very small so the CFSE in the intermediate is very small
1st row TM’s tend to go via 5 coordinate (dissociative mechanism) as they’re very small - hard to fit 7 ligands around the metal
2nd and 3rd row TM’s can do either (associative or dissociative) as they’re larger
crystal field activation energy
if the CFSE is very high in the starting complex then there is a big loss of CFSE in the intermediate, and this is an energy barrier to ligand exchange
CFAE is very large for d6 low spin complexes (Fe2+, Co3+) which means they are particularly kinetically inert towards ligand exchange
Zn2+ (d10) has no CFAE and exchanges ligands very rapidly - kinetically liable - ideal for catalysis
d6 low spin has the highest CFSE
what is the chelate effect
when k1 for a bidentate ligand > beta2 for a similar monodentate ligand eg. en vs NH3
two bonds are formed in each case but the formation of a chelate complex is more thermodynamically favourable
explaining the chelate effect
general
when the first bond is broken on a chelating ligand the ligand is still held near the metal and so will re-bond
the bidentate ligand is held more tightly and so more difficult to remove
entropy
delta G = delta H - T.deltaS
the stability of chelates relates to entropy ie. disorder in the system
the number of free molecules in the system is increased by forming the chelating system - 7 molecules rather than 4
entropy always favours the chelate complex
enthalpy is related to bond strength and can favour or disfavour the chelate
lone pairs
enthalpy may favour the chelate complex if both bonds are Ni-N amine as bonding the NH3 ligands created new lone pair repulsions where as with the chelate ligand the repulsions are already built in (in part)
effect of chelate ring size
5 membered > 6 membered > anything else
5 membered rings are the ideal and most stable
bigger than 6 means the binding atoms are too far away- compete with bulk solvent
exception to rule: acac - pseudo-aromatic 6 membered ring - very stable
the macrocyclic effect (not examined)
you get a further enhancement in binding if you join al the donors up in a ring as it means the ligands are still anchored in even if any bond breaks