Apex Inhaled Anesthetics 1 & 2
1/305
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
306 Terms
what is the blood:gas partition coefficient of nitrogen?
0.014 - you know this because N2O is 34x more soluble than nitrogen.
- since N2O is 0.46 → 0.46/34 = 0.014
- so for every 1 molecule of nitrogen that leaves a closed space, 34 molecules of N2O enter to take its place
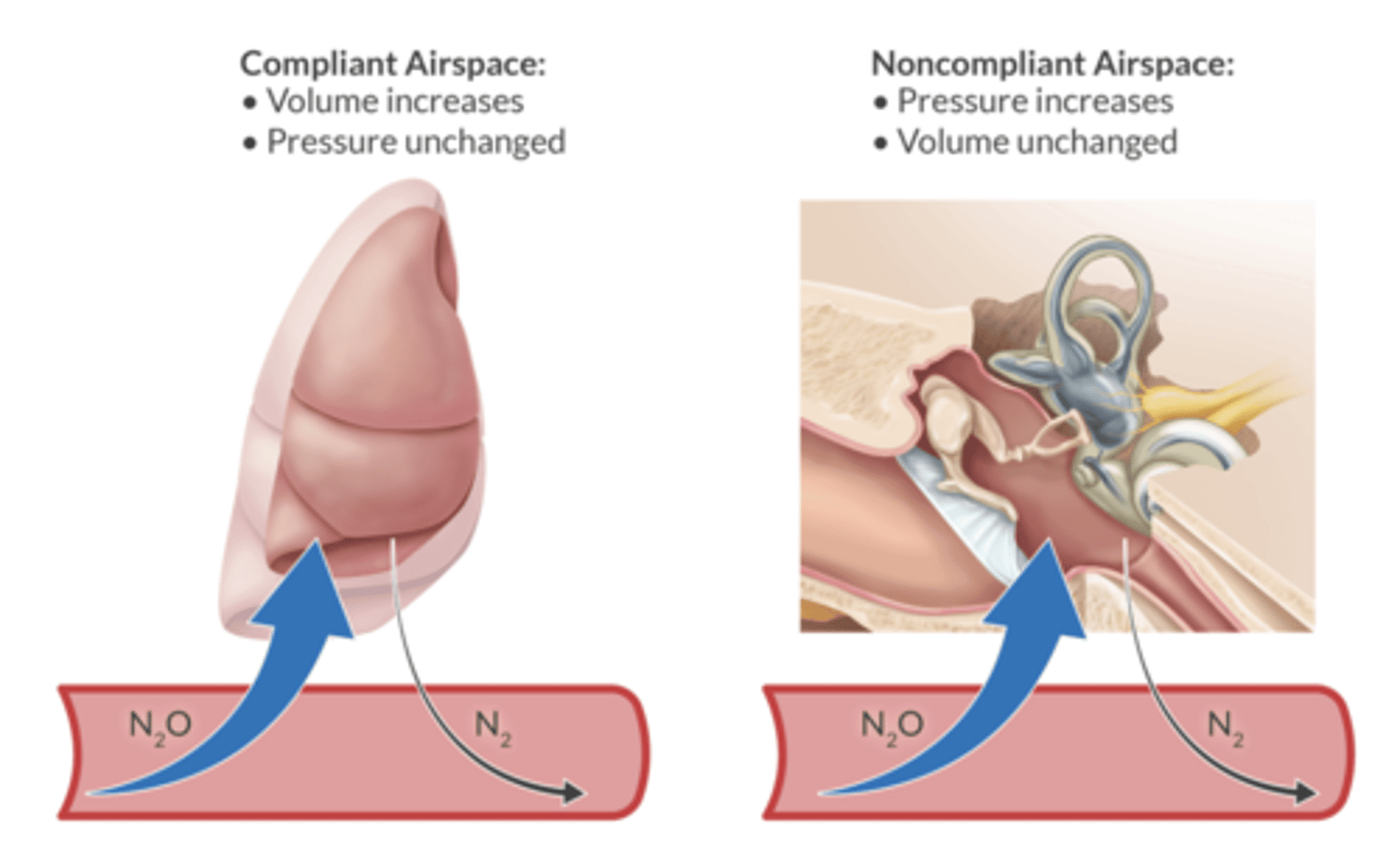
in a compliant airspace, what does N2O do to the voume of the space?
increase the volume - blebs, bowel, air bubbles in the blood.
in a fixed airspace, N2O increases the pressure of the space (middle ear, eye during retinal detachment surgery, brain during intracranial surgery)
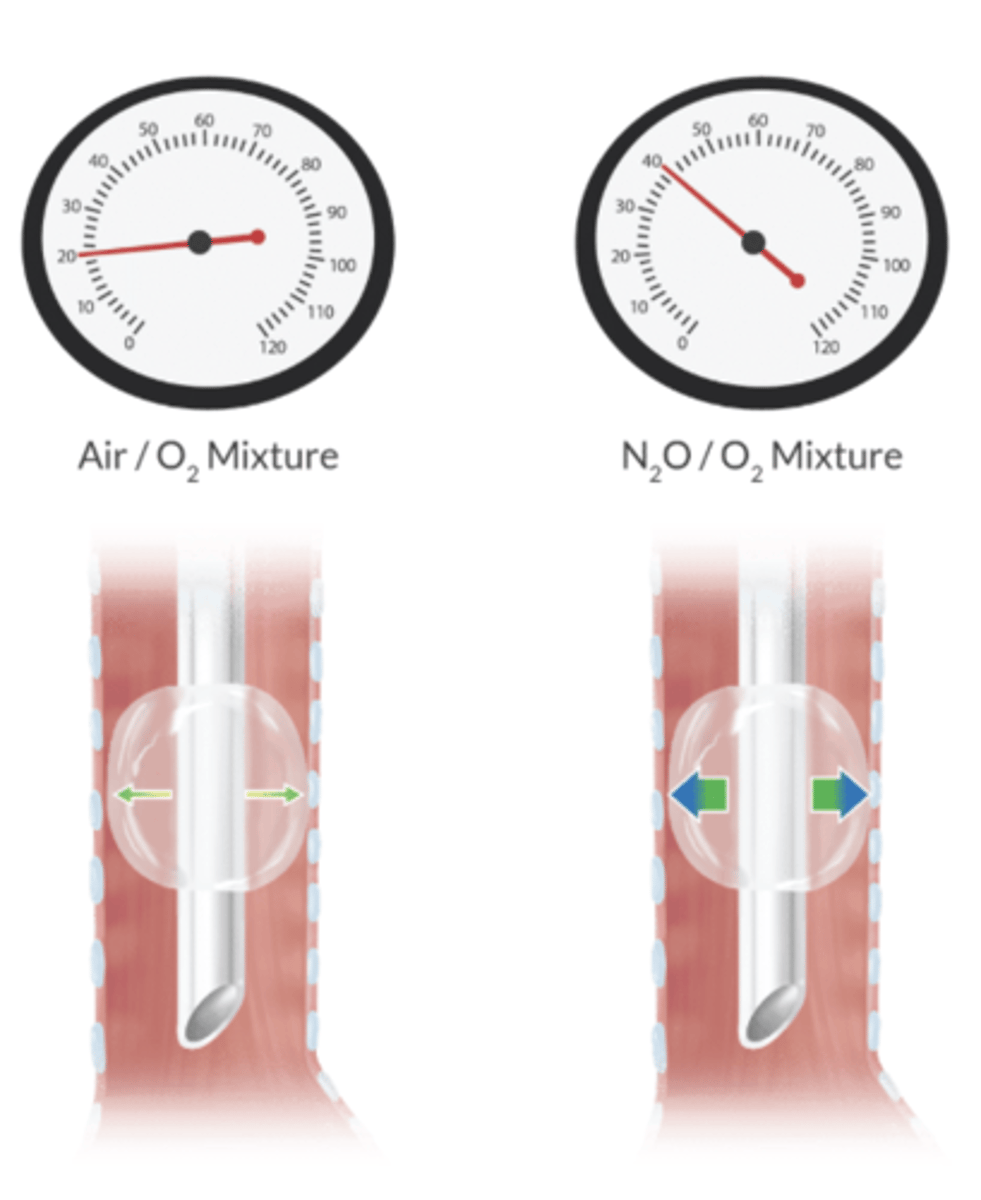
nitrous can increase the volume and pressure of what three pieces of anesthesia equipment?
ETT - so check with a manometer
LMA cuff
balloon-tipped pulmonary catheter
is N2O flammable?
non-flammable but does support combustion (as an oxidizer → when heated, it breaks down into N2 and O2 and the extra O2 feeds the fire or allows flammable agents to burn more vigorously)
there is a fire risk when N2O is administered during laparoscopy with pneumoperitoneum if electrocautery is used
which enzyme is inhibted by N2O?
irreversibly inhibits vitamin B12 → inhibtion of methionine synthase (an enzyme required for folate metaboism and myelin production)
of the compliant airspaces where N2O would increase the volume, which are fast vs slow equilibration?
fast = pulmonary blebs, air bubbles in the blood, gas bubble in the eye
slow = bowel, pneumoperitoneum
what are the two noncompliant airspaces wehre N2O would increase pressure?
fast equilibration between space and blood: middle ear, brain during intracranial procedures
what is the consequence of nitrous oxide quickly increasing the noncompliant middle ear pressure?
noncompliant → increased pressure → tympanic membrane graft damage
stopping N2O can quickly reduce middle ear pressure → serous otitis
what compound is placed over the site of the retinal break during retinal detachment surgery?
sulfur hexafluoride - this functions as a splint to hold the retina in place while healing occurs
- N2O can diffuse in, expand the bubble, compromise retinal perfusion and cause permanent blindness
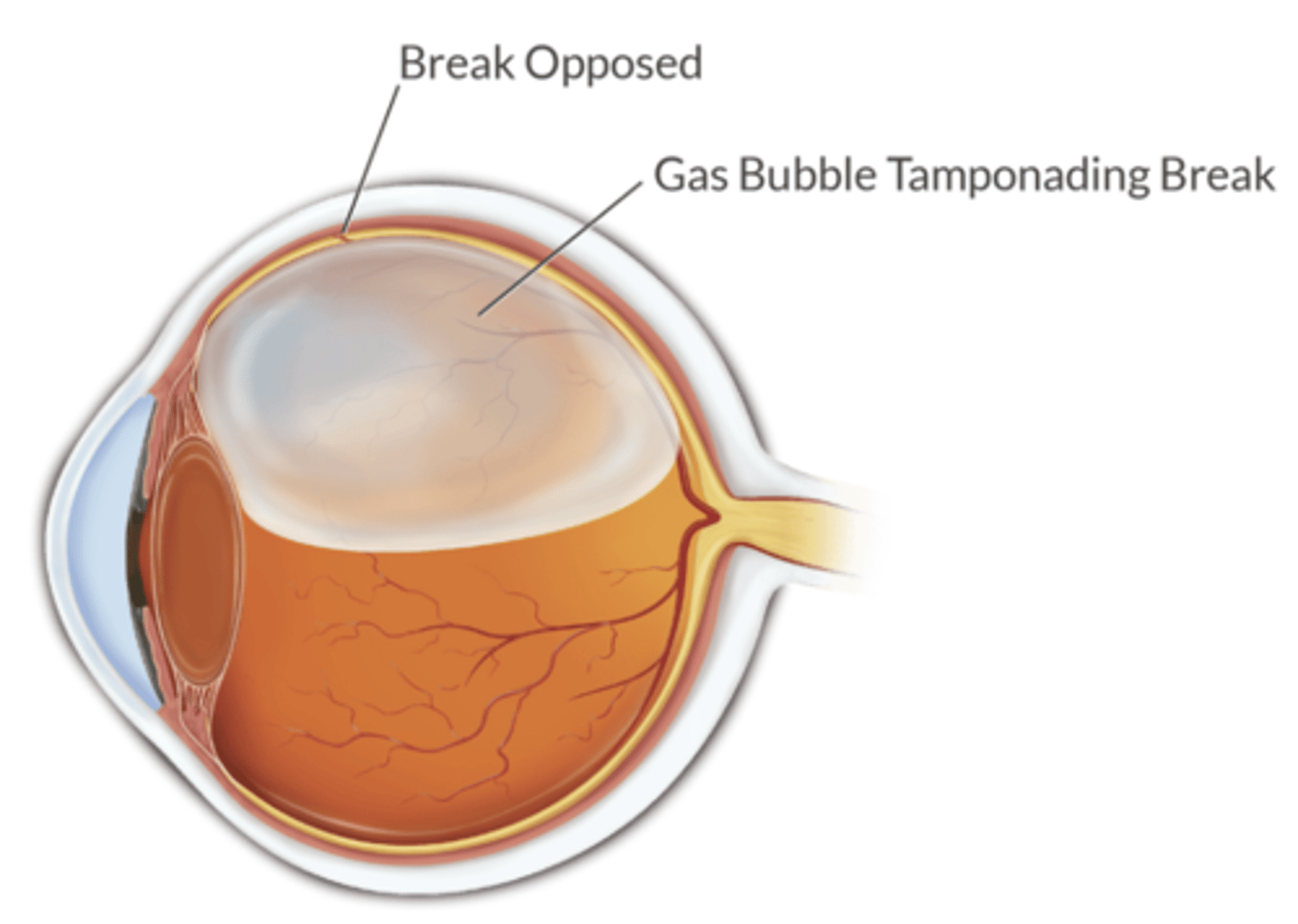
when should you avoid N2O in the setting of SF6 (sulfur hexafluroride)?
before SF6 is placed = discontinue N2O at least 15 minutes before placed
after SF6 is placed x 7-10 days
when should you avoid N2O in the setting of air, perfluorpropane or silicone oil bubbles?
air = 5 days
perfluoropropane = 30 days
silicone oil = no contraindication
what is the most reliable way to check the internal pressure of an ETT or LMA cuff?
manometer - palpation is grossly inaccurate
what are the potential side effects of N2O inhibiting vitamin B12 and thus, methionine synthase?
- reduced DNA synthesis
neuropathy
- megaloblastic anemia (bone marrow suppression)
- immunocompromised
- homocysteine accumulation
- teratogenicity
- spontaneous abortion (avoid in first two trimesters)
the risk of complications r/t N2O exposure is increased in what patients?
pre-existing vitamin B12 deficiency
pernicious anemia
alcoholism
strict vegan diet
recreational use of N2O
order the volatile agents from least to most potent
1. N2O (least)
2. Des
3. Sevo
4. Iso (most)
higher the MAC = lower the potency
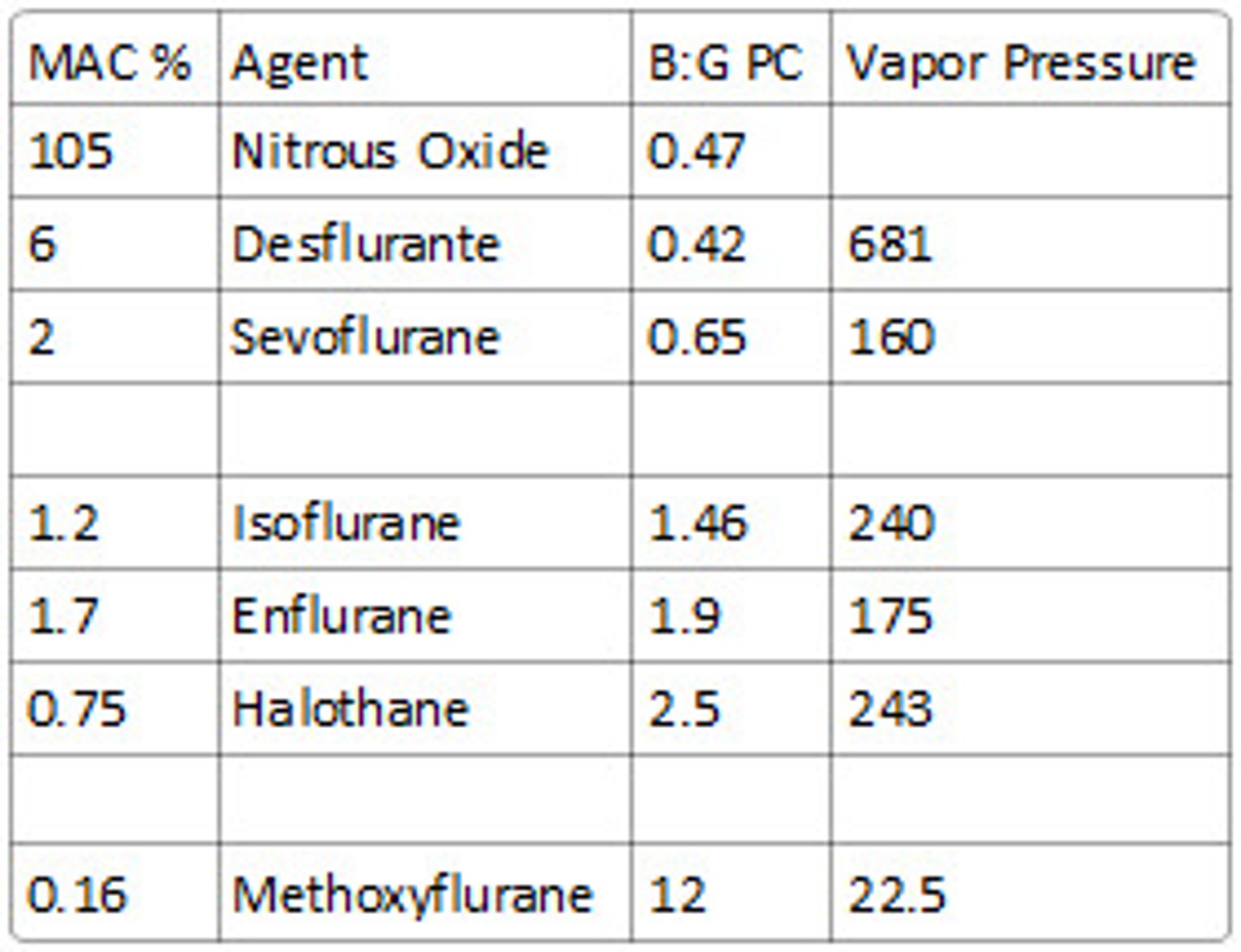
what is the MAC value of the volatile agents?
N2O = 104% = least potent
Des = 6.6%
Sevo = 2%
Iso = 1.2% = most potent
general anesthetics have what five effects?
- Amnesia
- loss of consciousness
- immobility
- modulation of autonomic function
- some analgesia
the essential triad = amnesia, LOC, immobility
Key MAC Facts
-MAC is additive
MAC-awake during induction
0.4-0.5 MAC
MAC-awake during emergence
0.15 MAC
MAC-bar (Minimum Alveolar Concentration for Blunting Adrenergic Responses)
1.5 MAC
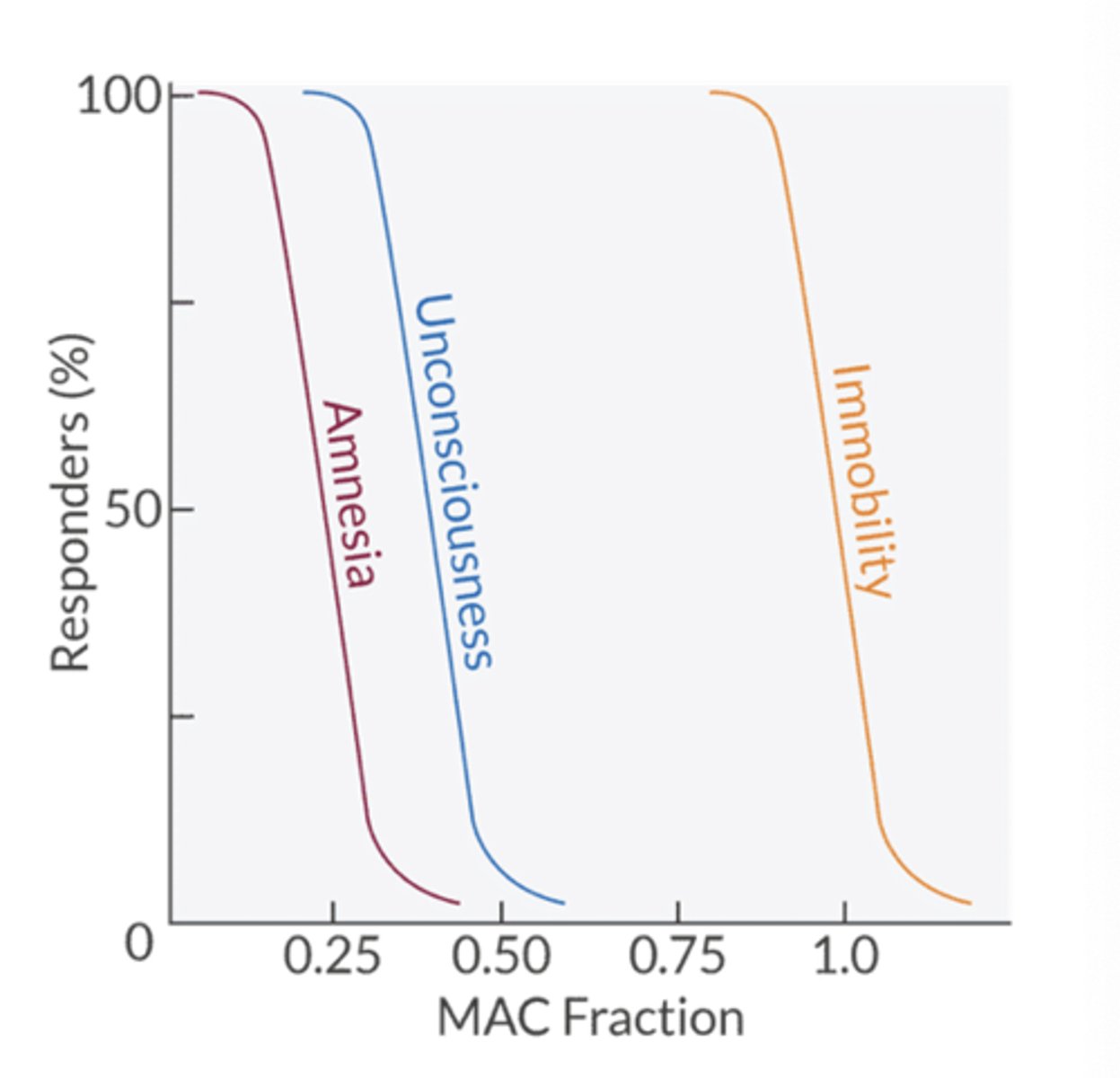
movement is prevented in 95% of patients at what MAC value?
1.3 MAC
awareness and recall are prevented at what MAC value?
0.4-0.5 MAC
What is a MAC hour
One times the MAC that prevents movement response to noxious stimulus in 50% of subjects administered for one hour
what factors increase MAC?
things that increase central nt concentration, neurotransmission and cerebral metabolism
- chronic alcohol consumption
- increased CNS neurotransmitter activity
- *hypernatremia
- infants 1-6 months
- hyperthermia
what factors decrease MAC?
- acute alcohol consumption
- sedative drugs (IV anes, N2O, opioids, alpha2 agonists)
- Lithium
- Lidocaine
- hydroxyzine
- hyponatremia
- old age (decreased 6% per decade after age 40)
- extremes of age
- pregnancy
what three factors have no effect on MAC?
hyper or hypothyroidism
hyper or hypokalemia
gender
what five drugs increase CNS neurotransmitter activity and thus, increase the MAC?
- acute amphetamine intoxication
- acute cocaine intoxication
- MAOIs
- Ephedrine
- Levodopa
what electrolytes affect MAC?
hypernatremia = increases
hyponatremia = decreases
how does body temperature affect MAC?
hyperthermia = increases
hypothermia = decreases
Hyperthermia raises neuronal excitability and increases cerebral metabolism (↑ CMRO₂).
Because neurons are more active, they require more anesthetic to achieve the same level of suppression.
how does red hair affect MAC?
increases by 19% (presumably due to mutation in melanocyte stimulating hormone receptor)
what physiologic processes reduce MAC?
- Hypotension (MAP < 50 mmHg)
- hypoxia
- anemia < 4.3 mL O2/dL blood
- cardiopulmonary bypass
- metabolic acidosis
- hypo-osmolarity
- postpartum (24-72hrs)
- PaCO2 > 95 mmHg
Causes of hypernatremia (which leads to increased MAC?
water loss
- DI
- osmotic diuresis (hyperglycemia, mannitol)
- GI losses (diarrhea, vomiting without fluid replacement)
- insensible losses (fever, sweating, burns)
sodium gain
- hypertonic saline admin
- NaHCO3 admin
- Cushings/hyperaldosteronism
MAC: increases → pts need more anesthetic because hypernatremia causes neuronal dehytration and irritability → CNS is more excitable
what causes of hyponatremia decrease MAC?
hypovolemic hyponatremia - GI losses, Renal losses (thiazides, Addisons), cerebral salt wasting
euvolemic hyponatremia - SIADH, adrenal insufficiency
hypervolemic hyponatremia - HF, cirrhosis, nephrotic syndrome, advanced renal failure
hyponatremia → lowers neuronal excitability and stabilizes membranes, CNS depression → pts need less MAC
how does hyper- and hypothyroid situations affect MAC?
not directly but changes in cardiac output can affect things
- reduced CO (profound hypothyroidism) → reduced VA uptake →faster rate of rise of FA/FI → faster induction
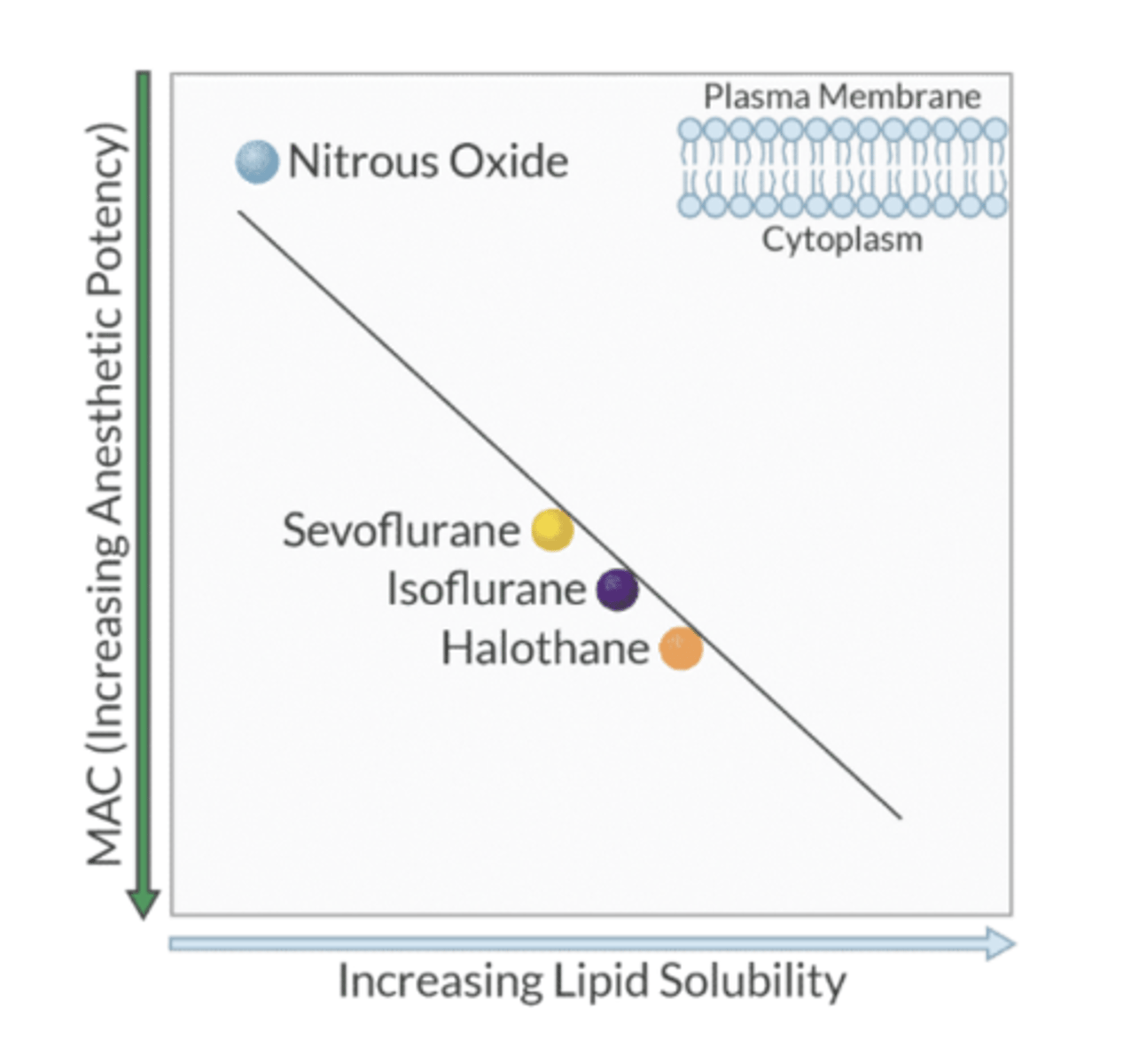
what is the Meyer-Overton rule?
lipid solubility is directly proportional to the potency of the inhaled anesthetic; depth of anesthesia is determined by the number of anesthetic molecules that are dissolved in the brain and not the agent used.
(The higher the lipid solubility, the higher the potency)
what is the unitary hypothesis?
all anesthetics share a similar mechanism of action but each may work at a different site
modern theory of anesthetic action suggests what two things?
1. Agents interact with stereoselective receptors - the stereoselectivity suggests a chiral binding site
2. agents produce immobility by binding in the dorsal horn of the spinal cord
they either stimulate inhibitory receptors or inhibit stimulatory receptors
what is the primary target of halogenated agents in the brain vs spinal cord?
brain = GABA-A (ligand gated Cl channel)→ stimulation causes increased Cl influx → hyperpolarization → impairs ability to fire. (VA increases duration that Cl channel remain open)
spinal cord = stimulate glycine channels and inhibit NMDA receptors, inhibit Na+ channels
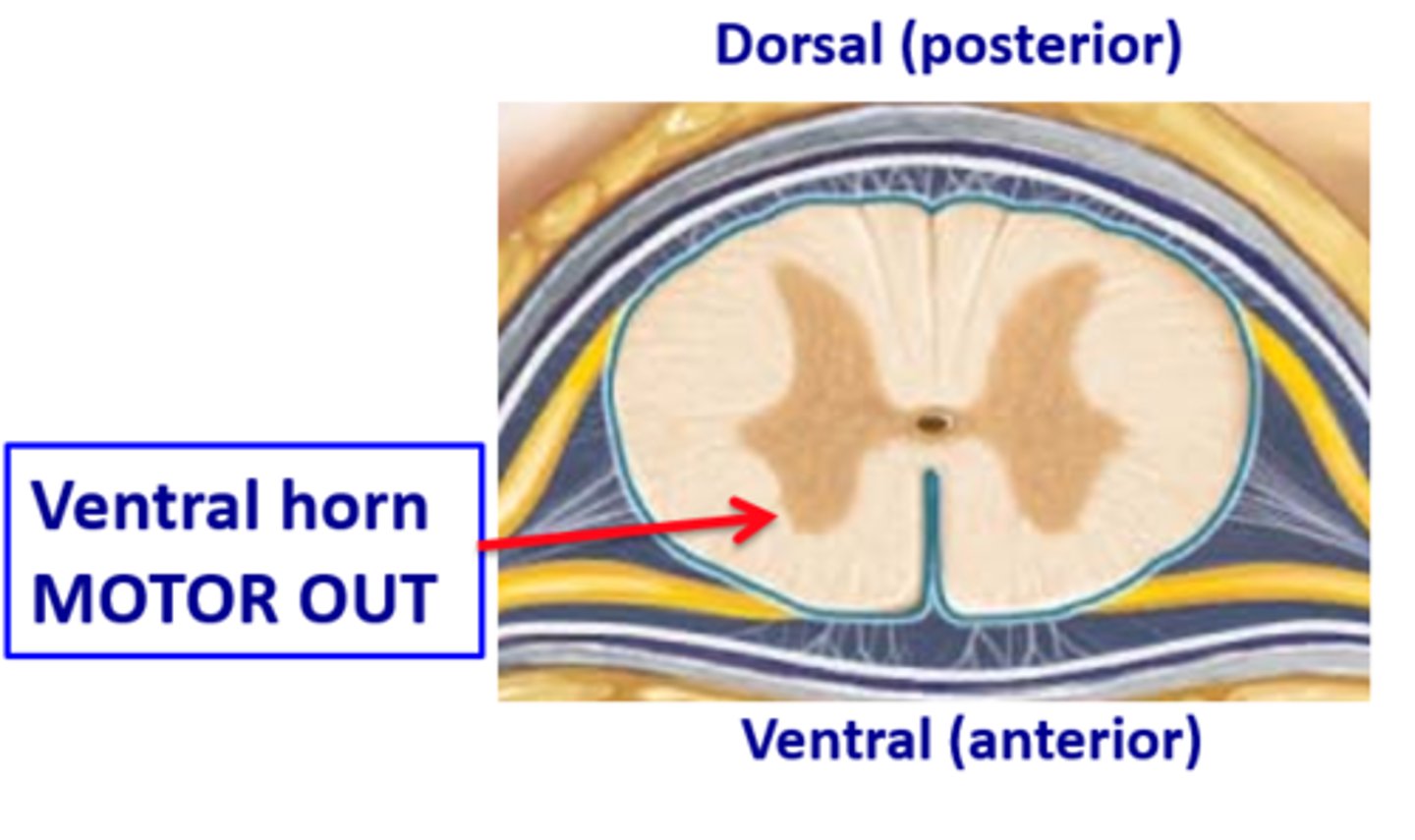
immobility is produced inthe ventral horn of the spinal cord
N2O and xenon target what two receptors/channels?
NMDA receptor - antagonism and potassium 2P channel (stimulation)
N2O + xenon do not involve GABA-A
what stimulatory pathways are inhibited by volatile agents?
NMDA receptors
Nicotinic receptors
Na channels
Dendritic spine function and motility
what inhibitory pathways are stimulated by volatile agents?
GABA-A receptors
Glycine channels
Potassium channels
do volatile agents increase the duration of Cl channels opening or the frequency?
duration that the channel is open
what are the three most important sites of volatile anesthetic action in the spinal cord?
glycine receptor stimulation
NMDA receptor inhibition
Na+ channel inhibition.
Note that immobility does not involve the GABA-A receptors in the spinal cord
how do volatile agents produce unconsciousness?
by interacting in the cerebral cortex, thalamus and RAS
cerebral cortex = higher order cerebral functions
The thalamus = relay for sensory and motor input to the cortex
RAS = consiousness and arousal
where is amnemia produced?
amnesia = amygdala and hippocampus
amygdala = emotion, pain, stress
hippocampus = memory
where are the autonomic effects of the volatile agents produced?
pons and the medulla - control center for autonomic reflexes
where is analgesia produced?
spinothalamic tract - nocicpetive signals along the ascending pain pathway are inhibited

where is immobility produced?
ventral horn of the spinal cord - upper and lower motor neurons synapse here
QT prolongation occurs at what MAC?
1.0 MAC = increases duration of myocardial repolarization by impairing an outward K+ current → prolonged QT
HR increases with which two volatile agents?
des and iso → 5-10% above baseline most likely d/t SNS activation from respiratory irritation
also, N2O
what are the CV effects of the volatile agents?
- dose-dependent MAP reduction
- dose-dependent decreased contractility (remains preload responsive)
- dose-dependent SVR decrease
which agent reduces SVR the least?
Sevo
which agent inceases MAP and SVR with SNS activation?
N2O
What protective benefit do volatile agents have on CV system?
myocardial preconditioning against ischemia
in cardiac and vascular smooth muscle, what is the impact of volatile anesthetics?
they reduce Ca2+ influx in the sarcolemma and reduce Ca2+ release from the SR
they also modulate NO release, inhibit ACh-induced vasodilation, impair Na/Ca pump to decrease intracellular Ca2+
which agent reduces HR?
Xenon
Sevo = no change
Des, Iso, N2O = increase
which agent reduces BP the most?
Iso
Xenon = no change
what two agents may or may not reduce CO?
Des, Sevo
xenon = no change
Iso, N2O = decrease
all agents reduce SVR except which one?
N2O
xenon = no change
MOA: decreaesd intracellular Ca2+ in vascular smooth muscle → systemic vasodilation → decreased SVR
sevo reduces SVR the least
what is the primary cause of MAP reduction?
decreased intracellular Ca2+ in vascular smooth muscle → systemic vasodilation → decreased SVR, decreased venous return
secondary cause: decreased intracellular Ca2+ in the cardiac myocyte → myocardial depression → decreased inotropy
how do halogenated agents affect HR?
increase = des, iso, N2O
- decreased SA node automaticity
- decreased conduction velocity through the AV node, His-Purkinje system and ventricular conduction pathways
- increased duration of repolarization by impairing outward K+ current → increased action potential duration → prolonged QT interval
- altered baroreceptor function
what is the mechanism by which rapid increases in desflurance can cause tachycardia?
pulmonary irritation → SNS activation → incrased Ne release → beta-1 stimulation
tachycardia can be minimized with opioids, alpha-2 agonsits, beta-1 antagonsits
combining N2O with what medication can cause myocardial depression?
N2O + opioid
how do volatile anesthetics affect coronary blood flow?
increases;
RT reduction of myocardial O2 demand → small cardiac vessels (20-50 micrometers in diameter) dilate
iso > Des > sevo
which agent is the most potent coronary artery vasodilator?
Iso
but doesnt contribute to cornary steal
how do halogenated agents affect the respiratory rate and rhythm?
Halogenated (usually ethers or alkanes) have halogen atoms — fluorine, chlorine, or bromine.
Ex:
Halothane (contains bromine, chlorine, fluorine)
Isoflurane, Sevoflurane, Desflurane (contain fluorine)
reduced tidal volume → increased RR → increased dead space ventilation
bronchodilators → increased airway diameter → decreased resistance
MV decreases → TV gets smaller + partial compensation by increasing RR
what effects do the halogenated agents have on PaCO2?
- hypercapnia d/t depression of central chemoreceptors and respiratory muscles
- altered respiratory pattern (fast and shallow)
- increasd apneic threshold
- relaxation of muscles that maintain upper airway tone → airway obstruction
- bronchodilation
- decreased FRC
for every 1mmHg increase in PaCO2 above baseline, how will Mv change?
1mmHg increase in PaCO2 → increased Mv by 3L/min
what are the three mechanisms by which a dose-dependent depression of the central chemoreceptor and respiratory muscles contribute to hypercarbia?
1. altered respiratory pattern
2. impaired response to CO2
3. impaired motor neuron output and muscle tone to the upper airway and thoracic muscles
the CO2 response curve shifts in what direction in the setting of halogenated agents?
decreased response to CO2 → down and to the right
other causes of this shift: opioids, metabolic alkalosis, denervation of peripheral chemoreceptors
the right shift implies taht for a given PaCO2, the Mv is less than predicted → creates a respiratory acidosis
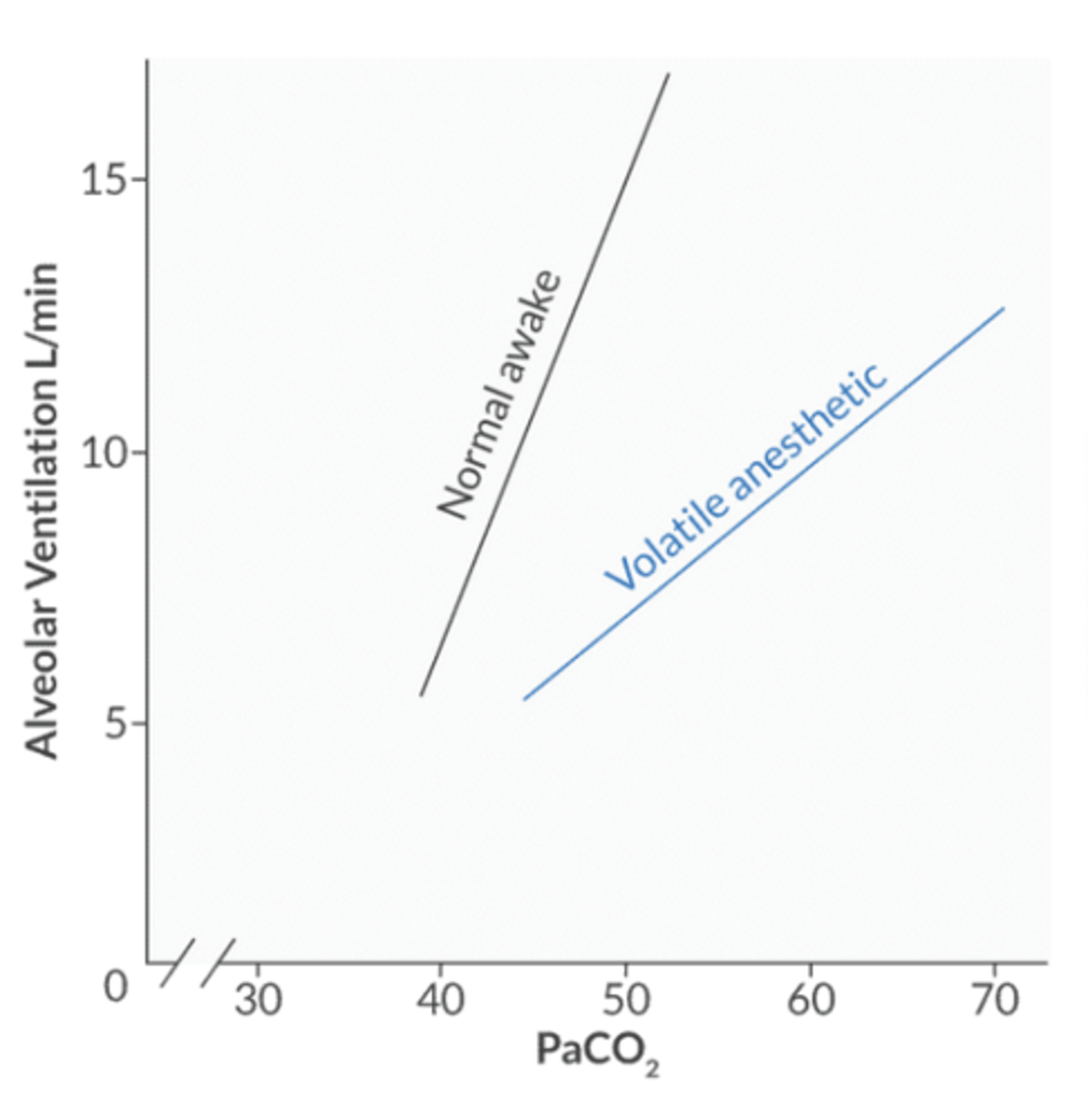
what factors cause a left shift of the CO2 response curve to stimulate ventilation?
anxiety
surgical stimulation
metabolic acidosis
increased ICP
salicylates
aminophylline
doxapram
the left shift implies that for a given PaCO2, the Mv is greater than predicted → this creates a respiratory alkalosis
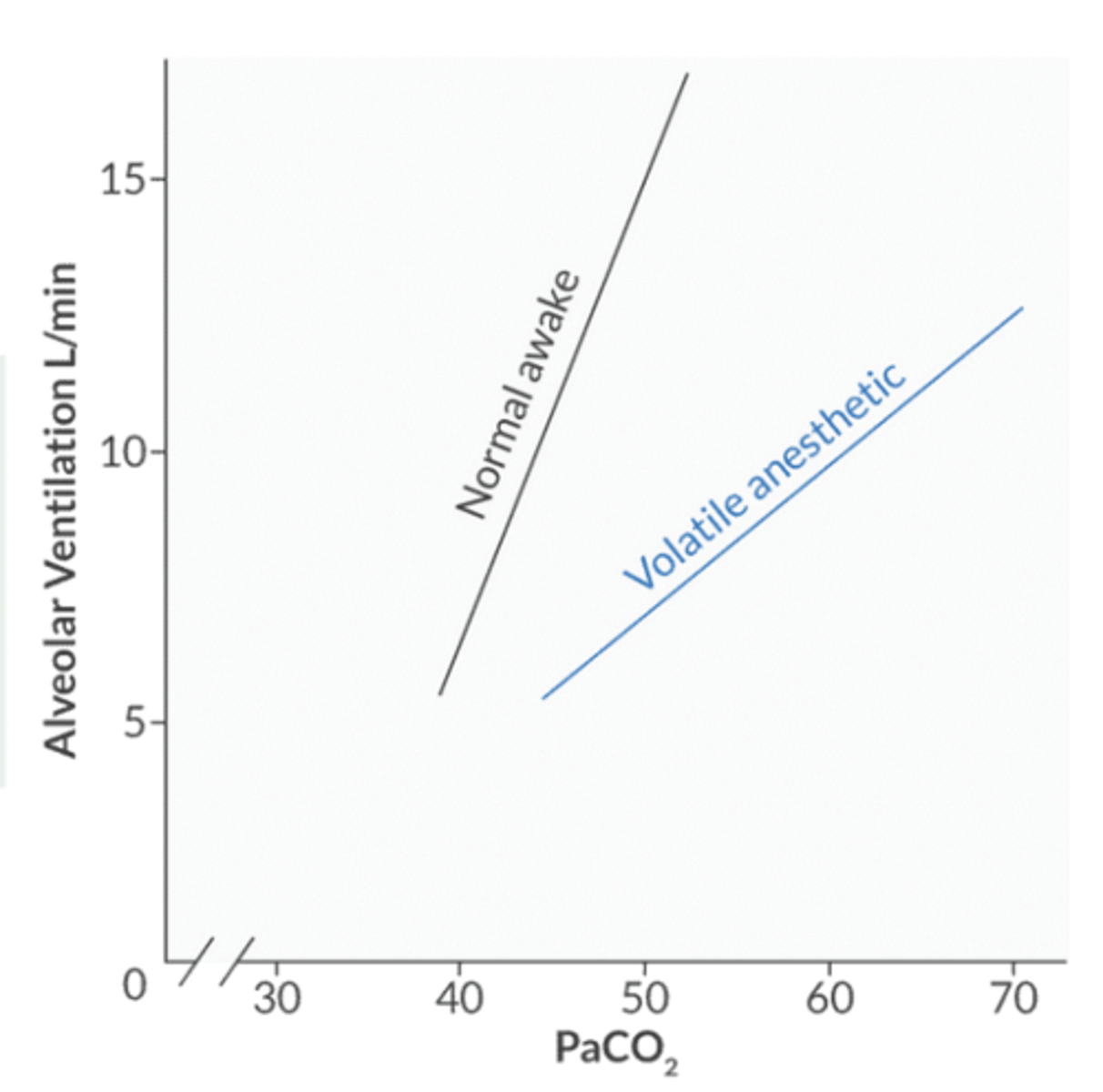
Do volatile agents increase or decrease the apneic threshold?
increase → PaCO2 at which the patient is stimulated to breathe
volatile agents impair what muscles → upper airway obstruction?
genoglossus or tensor palatine
what are the two consequences of impaired pulmonary muscles in the setting of volatile anesthetic?
- decreased FRC
- ineffective ventilation
which volatile agent can induce bronchoconstriction in asthmatics?
desflurane
which volatile agent impairs HPV the least?
des
how do halogenated anesthetics affect PaO2?
- impaired peripheral chemoreceptors (increased r/f hypoxemia)
- Impaired response to acute hypoxemia occurs at 0.1 MAC
- des impairs HPV the least → best choice for pts who rely on hypoxic drive to breathe (emphysema or OSA)
do pain and surgical stimulation reverse depression of the hypoxic ventilatory drive?
no
which chemoreceptors monitor for hypoxemia and are important in the hypoxic ventilatory response?
peripheral chemoreceptors in the carotid bodies
PaO2 < 60 mmHg is a stimulus that increases Mv to restore arterial oxygenation
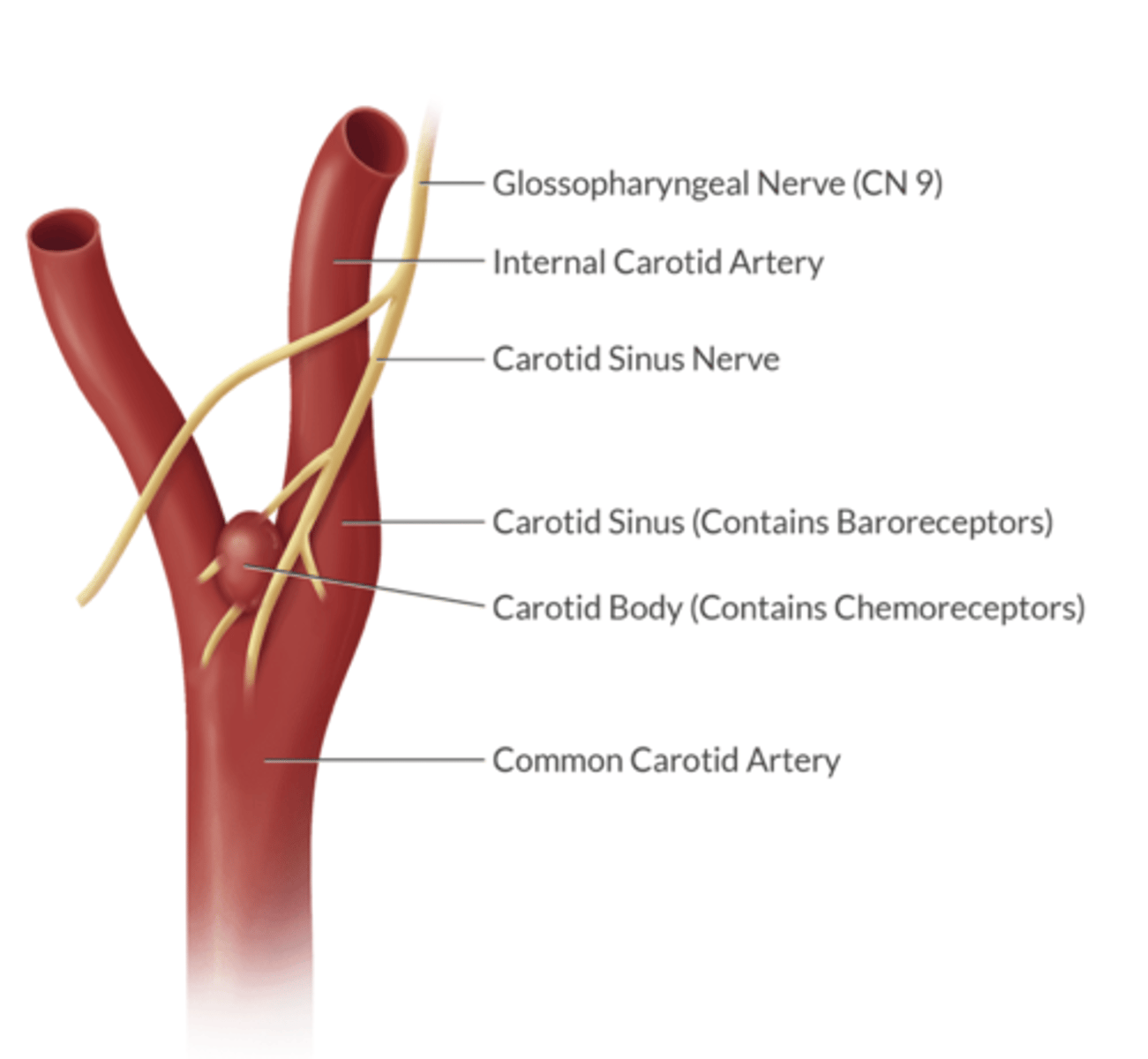
whare are the afferent limbs of the carotid and aortid body baroreceptor reflex?
carotid body = glossopharyngeal n. 9
aortic body = vagus n. 10
which is more sensitive to changes in arterial gas tensions (PaO2, PaCO2 and H+): carotid bodies or aortic bodies?
arterial gas tensions = carotid bodies
changes in blood pressure = aortic bodies

volatile agents depress ventilation by inhibiting muscle function in what three areas?
1. upper airway
2. diaphragm
3. intercostals
for what length of time do volatile anesthetics impair the peripheral chemoreceptors response?
up to several hours after anesthesia
impaired response to acute hypoxia can occur at what MAC?
0.1 MAC
but 0.1 MAC does not impair the response to PaCO2
why cell type in the carotid bodies provide the sensory arm of the hypoxic drive?
glomus type 1 cells - hypothesized that volatile agent metabolism creates a reactive oxygen species that impairs the glomus type I cells
so, the agents that undergo the greatest amount of biotransformation (hepatic metabolism) inhibit the hypoxic drive the most (Halothane > Sevo > Iso > Des)
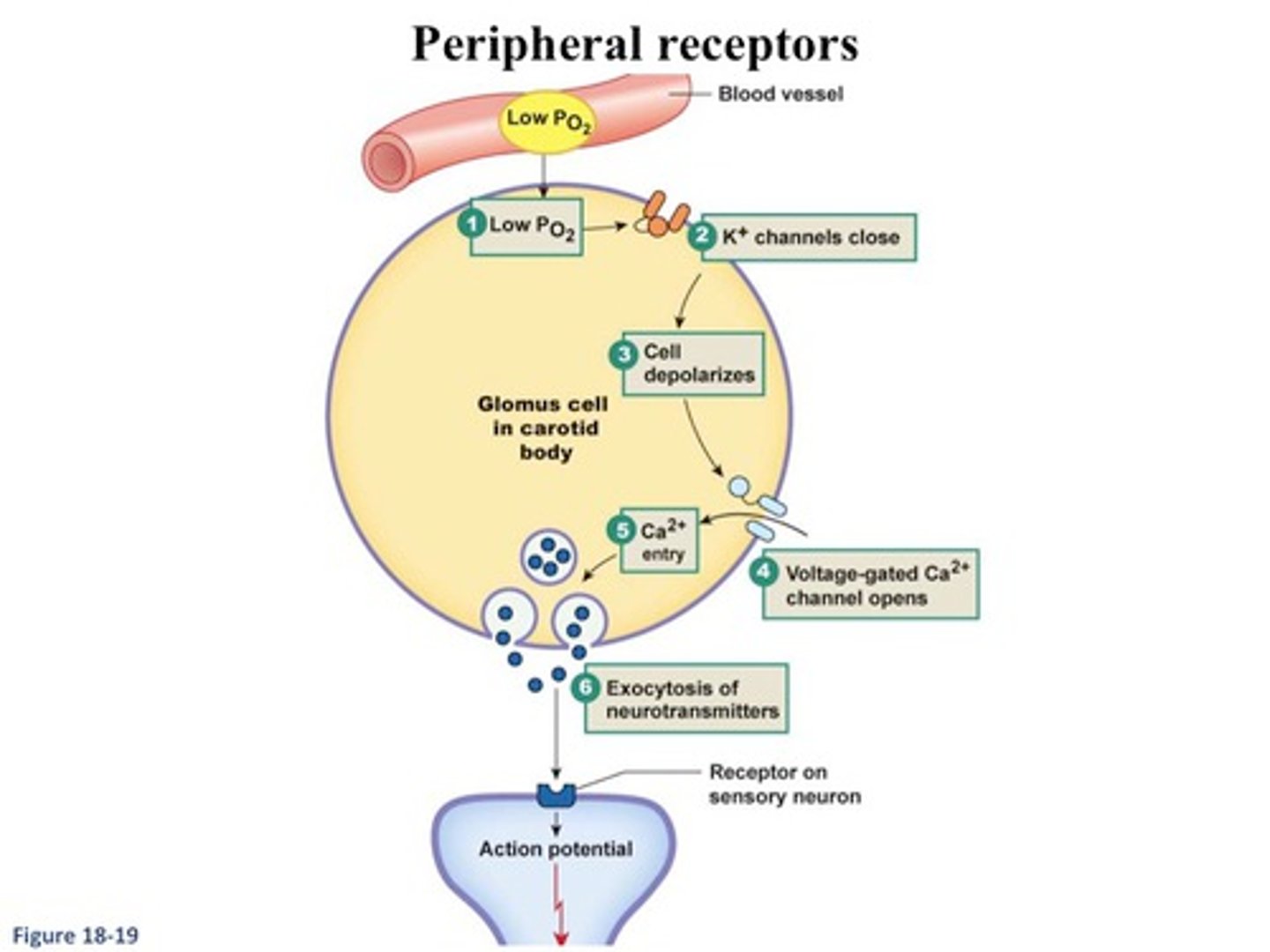
does N2O impair the carotid body response to hypoxemia?
yes, but by a different mechanism than the volatile agents - acts at glomus cell membrane channels to inhibit excitatory neurotransmission from the glomus cells to the afferent fibers of the carotid sinus → reduces dopaminergic and ACh-mediated signaling that normally increases in hypoxia
the carotid sinus n. is a branch off of what cranial nerve?
CN 9
where are the carotid baroreceptors located?
in the carotid sinus
what are the neurophysiological effects of halogenated anesthetics?
dose-dependent reduction in CMRO2
dose-dependent increase in CBF, CBV and ICP
this is known as uncoupling
at what MAC can Sevo produce seizure activity?
2.0 MAC - exacerbated by hypocapnia and is more common in pediatrics
how does N2O affect CMRO2 and CBF?
increases both
CMRO2 depends on what two variables?
1. electrical activity (60% of total brain oxygen consumption)
2. cellular homeostasis (40% of total brain consumption)
how do volatile agents affect CMRO2?
reduce CMRO2 but only to the extent that they reduce electrical activity.
- once the brain is isoelectric, volatile agents cannot reduce CMRO2 any further
what MAC of volatile agent is required to produce an isoelectric state?
1.5-2.0 MAC
- can reduce MCRO2 by ~60%
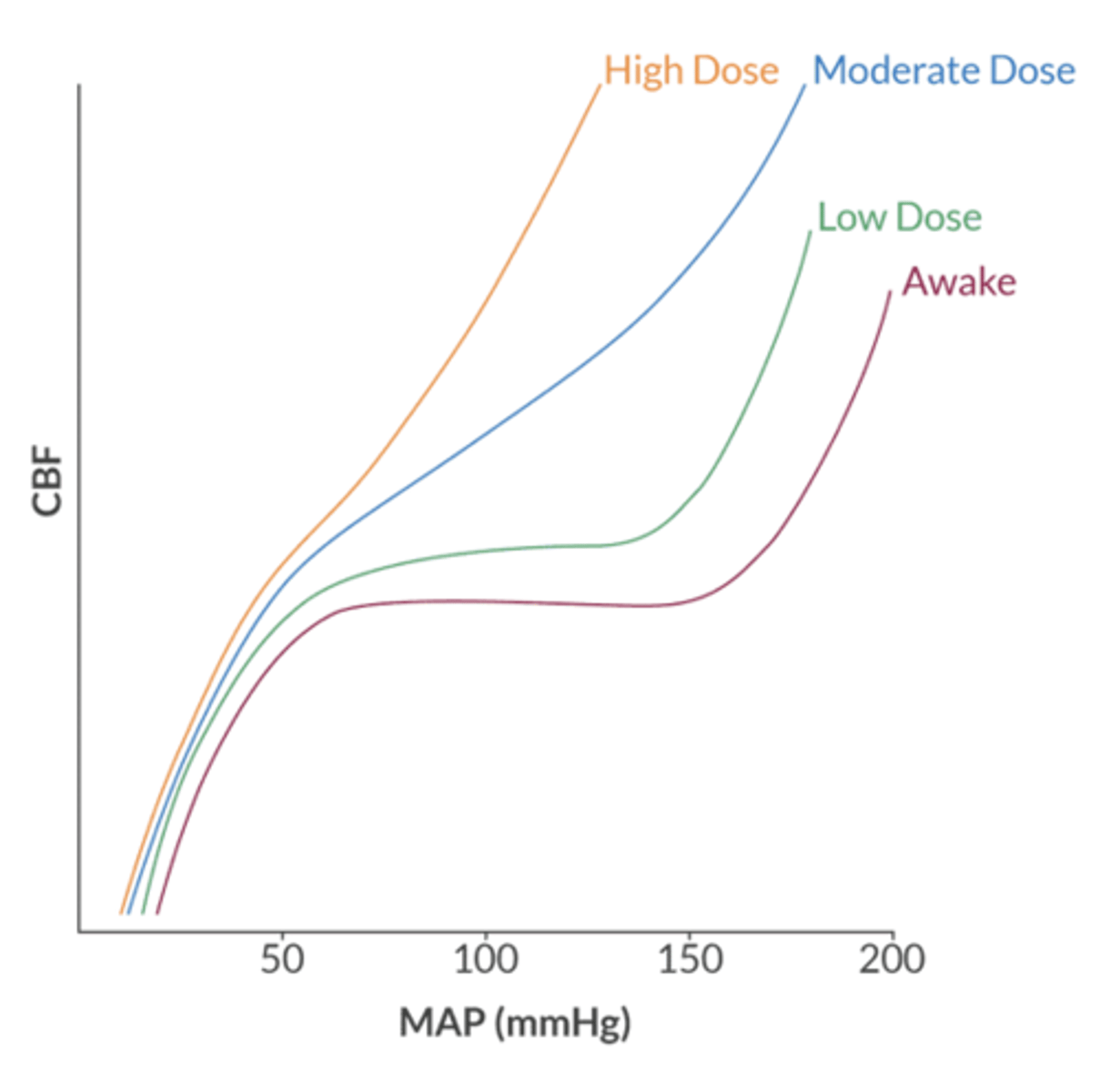
what is the normal physiology of cerebral blood flow?
autoregulation according to metabolic requirements
- metabolic demand increases → blood vessels dilate → reduced resistance → increased CBF
- metabolic demand decreases → vessels constrict → resistance increases → CBF decreases
how do volatile agents affect CBF?
volatile agents = cerebral vasodilators by reducing cerebrovascular resistance
- vasoconstriction from CMRO2 reduction
- vasodilation from anesthetic agent
CBF and CMRO2 uncoubling occurs above what MAC of volatile agents?
0.5 MAC = increase CBF and decrease CMRO2
- upside = favorable cerebral oxygen supply-demand ratio
- downside = increased ICP (problem in the setting of intracranial HTN)
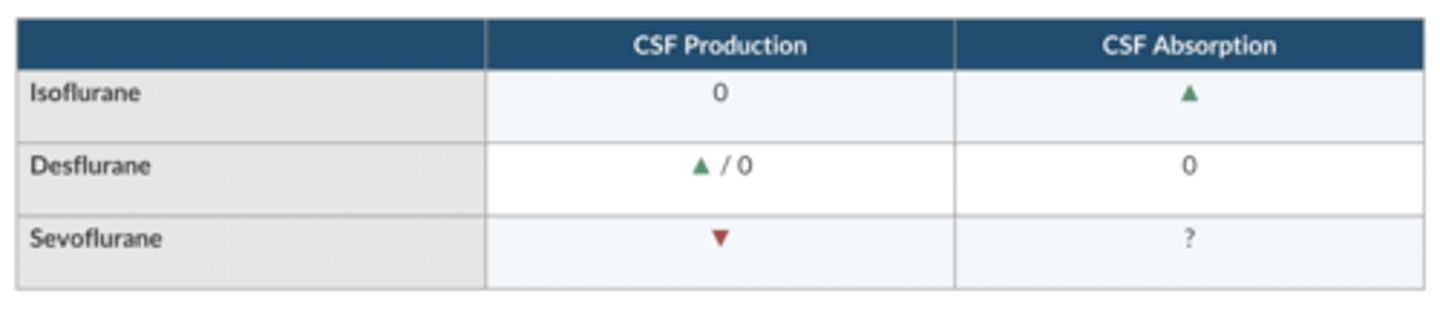
which volatile agent increases CSF absorption?
Iso
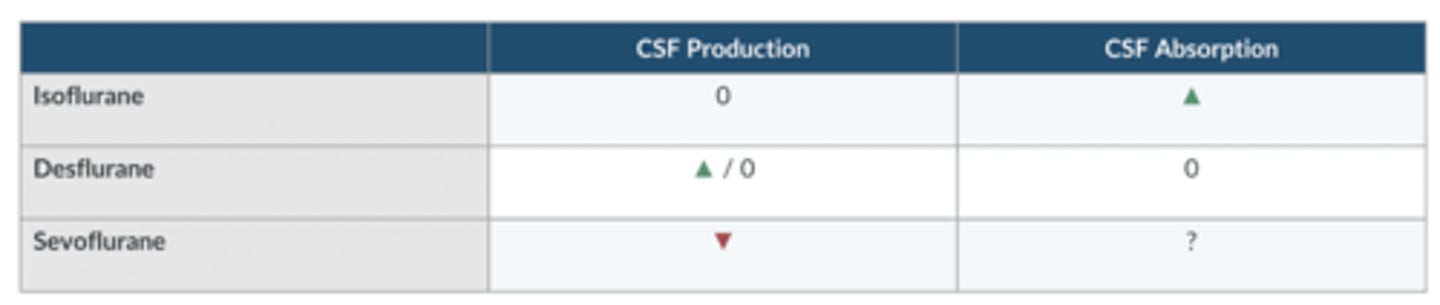
which volatile agent increases CSF production?
Des