bimm 120 -- final
1/88
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
in regard to symbiosis, define the following?
mutualism
commensalism
parasitism
mutualism: both benefit
commensalism: one benefits, other unaffected
parasitism: one harmed
in regards to origin of human microbiome, what was the original hypothesis of the origin but what has been evidently found? what does this indicate?
hypothesis: sterile womb
actual: womb x sterile
bacteria in umbilical cord blood, amniotic fluid, fetal membrane
no inflammation
indicate: infant incorporate initial microbiome before birth; birth/feeding add onto
in regards to microbial transmission from mom to child, where does the mother’s mouth/intestine/vagina bacteria end up in the child?
mouth → amniotic fluid
transmitted via bloodstream
Intestine/oral → meconium (baby first poo)
meconium contain enterobacteriaceae + lactic acid bacteria
vagina/bloodstream → womb
vagina
during pregnancy, vagina microbiome ↑ lactobacillus (acidic pH)
vaginal microbiome differs btwn dif ethnicities
what is the meconium? what is it indicative of?
meconium: first bowel movement of newborn
means body = functioning
what is jimenez et al study do to the meconium? what did they find?
21 newborn meconium samples
found not sterile
e. faecium → orally pregnant mice
e. faecium found in meconium
are bacteria pH sensitive? what pH are human symbiotic bacteria? molds/yeasts?
yes; human symbiotic/pathogenic = 6.5-7.5 pH; molds/yeasts = 4-6 pH
how does the vaginal pH change? what does that do?
acidic pH (<4); prevents candida albicans growth (> 4-4.5 pH) → prevents UTI
pH < 4 → ↑ lactobacilius (pregnant woman)
what expt did dominguez-bello et all study in terms of newborns and delivery? what was their findings? conclusion?
study: 16 rDNA sequencing study microbiome newborns; vag delivery/c-section
tried to restore microbime
found: c-section → mom skin microbiome; vaginal → mom vag
after 1 yr, microbiomes converge
conclusion: mode of delivery shape acquired/initial newborn microbiota composition
what 2 types of bacteria do vaginal born babies have?
lactobacillus; digest lactose (milk sugar)
bifidobacterium; prebiotic in breast milk break down human oligosaccharaies → short chain fattty acids → baby uses
what 1 type of bacteria do c-section born babies have? how long does it take to appear?
bacteroidetes; 6mo
what does breast milk contain and what does it do to bifidobacteria?
breast milk has urea — bifidobacterium → nitrogen → baby uses
what is the first milk the mother makes called? what is it rich in?
Colostrum; rich in urea + milk oligosaccarides
maturing babies: colostrum ↑ urea
what bacteria gets transferred from mom breast skin to the newborn?
sebaceous skin → breast milk
staphylococcus, corynebacteria, propionibacteria
what bacteria gets transferred from mom breast milk to the newborn?
mammary gland (lymph circulation) → baby oral cavity
streptococcus, staphylococcus, serratia, lactococcus
after how long after birth does vaginally and c-sectoin newborn’s microbiomes converge? why does it take this long?
1 year; starts differently
1 year bc start eating solid foods
what 2 bacteria dominate the adult gut?
bacteroidetes
firmicutes phyla
what are the 3 roles of microbiomes?
first line of defense
immune system educator
metabolism
what happens if baby is given honey? what is in honey that causes this?
clostridium botulinum → infant botulism
honey: endospores → germinate intestine and crease hypErtonic environ
c. botulinum toxin —| muscle contract → flaccid paralysis
baby small microbiome + x bile = x fight
what do normal microbiota produce to kill other bacteria?
bacteriocins
what 2 strategies have pathogens evolved to overcome normal microbiota?
use other nutrients
use other niches (via virulent factors)
what 2 things does the normal microbiota produce to protect itself from pathogens?
Iga (antibodies)
mucus; protect intestine from pathogens + used as carbon source by gluconate
what differs btwn germ free mice and regular mice? how can this be restored? waht recognizes these restoration factors? what do they trigger?
germ free mice = thinner mucus layer
restore: ↑ LPS + peptidoglycan (PGN)
TLRs (toll-like- receptors)
activate immune system
waht is the order of the central dogma?
DNA → RNA → protein
how are genes expressed in bacteria?
alter DNA seq
transcription intiation
control mRNA stability
translation control
sense environment
quorum sensing
2 component signal transduction
what are constitutive genes? inducible genes>
constitutive genes: housekeeping genes; always on (ie. glycolysis proteins)
inducible: only need in certain environments (ie. B-galactosidase, sporulation enzymes)
↑ nucleotides in space: ↓ RNA pol bind
is lactose and glucose mono/disaccharides? what is prefered by e.coli?
lactose: disaccharides
glucose: monosaccharides
fewer steps to break down, e.coli prefer
what is an operon? promoter?
operon: DNA segment w several genes controlled by promoter
promoter: where RNA pol will bind (-35/-10)
operon + promoter share same region → crucial for lac operon expression
what are the 5 parts on lac operon? waht do the 3 code for?
lacl: lac repressor (not part of lacO)
→ bind/loop DNA → prevent RNA pol bind x access promoter
promoter
lacO: operator
3 genes
lacZ: B-galactosidase (lactose → 2 glucose)
lacY: lactose permease (lactose entry)
lacA: galactoside transcetylase (lactose isomers)
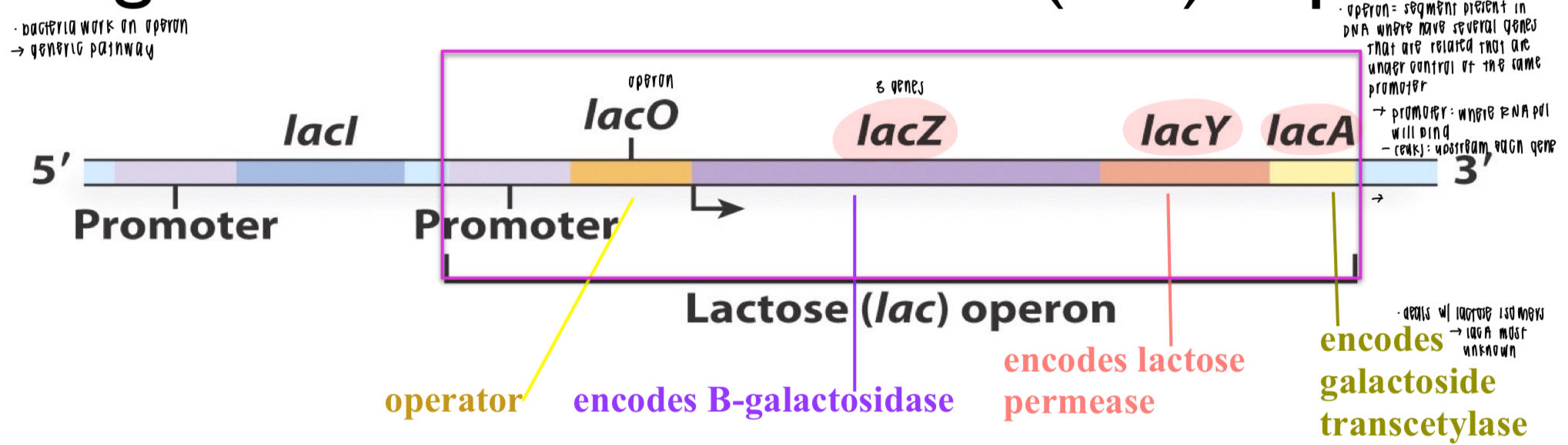
will the distuption of one gene on lac operon affect the others? why? what does this allow?
each gene own start/stop site
polycistronic pre-mRNA
(— trans splicing → monocistronic mRNA)
allows ↑ diversity/gene expression
how does lacI work in terms of repression? (-)
lacl → lactose repressor
binds to operator; bends DNA into loop
RNA pol x bind to promoter; prevent lacO operation
what represses the repressor of lacO?
allolactose bind to repressor — conformational change → repressor cant repress operator
lacO transcribed
what repression is responsible for lacO prioritizing glucose > lactose when both are present?
glucose = monosaccharide
catabolite repression: regulation of transcription by repressors/activators
induces diauxic growth
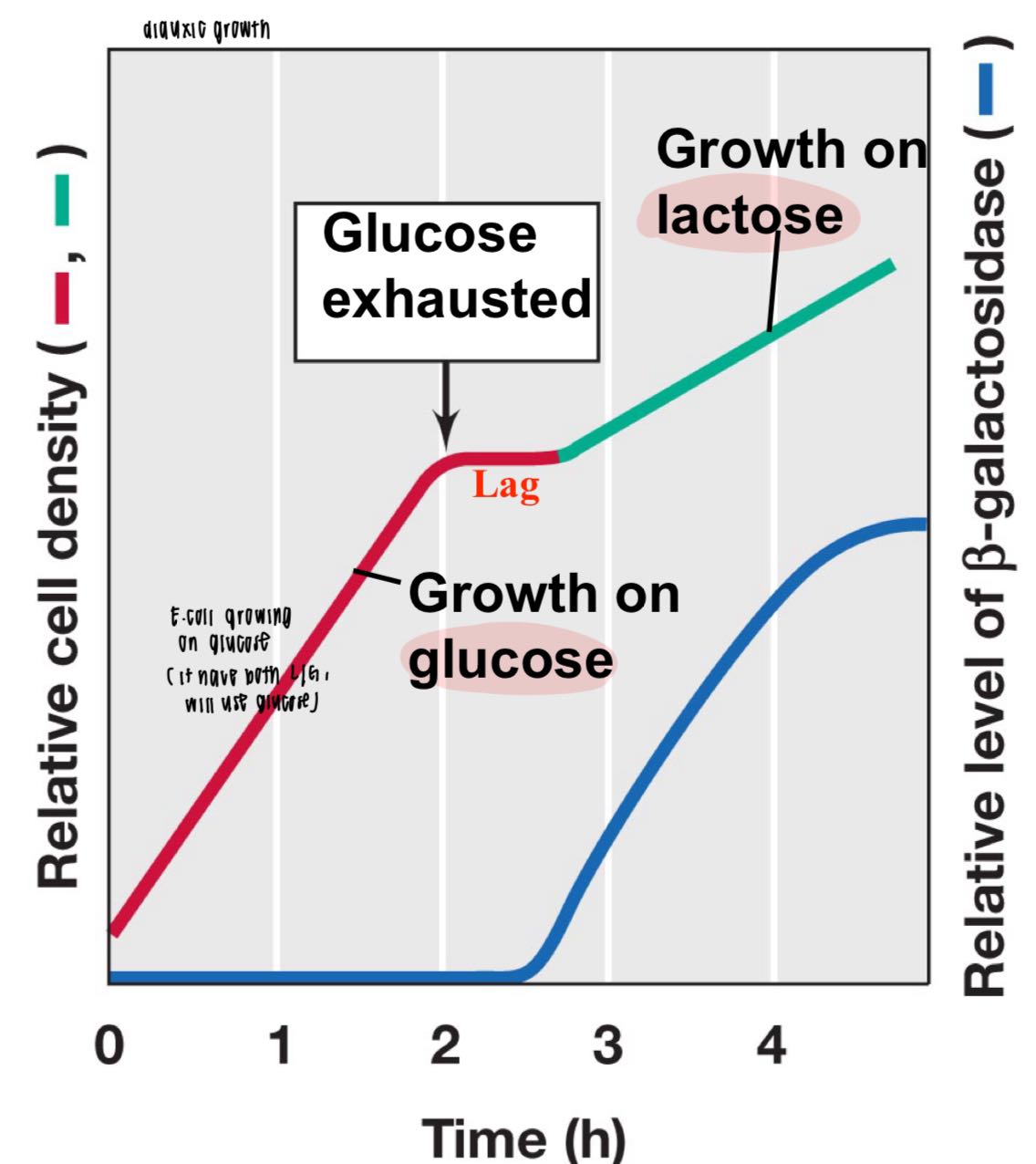
what is diauxic growth? why does a lag occur?
diauxic growth: biphasic growth pattern
both availble, glucose > lactose pref
lag occurs after glucose used up to switch to lactose
CAP/CRP; catabolite activator protein
activated by cAMP (upstream RNA pol)
what regulates cAMP levels?
↑ PEP & sugar phosphotransferase system & AC: ↑ cAMP
glycolysis → PEP → pyruvate
(glucose present) PEP transfer phosphoryl group → new glucose molecule
AC active = little/x glucose
what regulates CAP activity? what does CAP do?
CAP: bends DNA allow spacer to ↑ -10 region + RNA pol binding
↑ cAMP; ↑ CAP activity; ↑ lacO
little/no glucose = AC active
AC + PEP = ↑ cAMP: ↑ CAP
high glucose + CAP inactive (no cAMP)
what happens to lacO if there is no lactose present?
no allolactose made
if ↑↑↑ glucose, ↓ cAMP, x lactose, will lacO be activated?
no lactose: no allolactose to stop lacI repressor (no matter glucose levels)
high glucose: AC inactive = ↓ cAMP = ↓ CAP
no gene expression (x B-galactadase)
if ↓↓↓ glucose, ↑↑↑ cAMP, x lactose, will lacO be activated?
no lactose: no allolactose to stop lacI repressor (no matter glucose levels)
low glucose: AC active = ↑ cAMP = ↑ CAP
no gene expression
if ↓ glucose, ↑↑↑ cAMP, yes lactose, will lacO be activated?
yes lactose: allolactose stop lacI repressor
low glucose: AC active = ↑ cAMP = ↑ CAP
high gene expression
who discovered the first antibiotic? which was it? are they naturally producing?
fleming → penicillin (mold)
antibiotics: chemical compounds naturally produces by bacteria/fungi to compete other microorganisms
have selective toxicity
what is selective toxicity? what 2 groups could antibiotics be?
ability of drug kill/inhibit pathogen wo affect host
-static: inhibit growth
-cidal: kill bacteria
antibiotics: broad/narrow spectrum
what class of antibiotics degrade cell wall? how is this selectively toxic?
b-lactam antibiotics
↓ peptidoglycan crosslinking
aka transpeptidation (x cell wall synthesis)
animals x have cell wall; gram(+) only
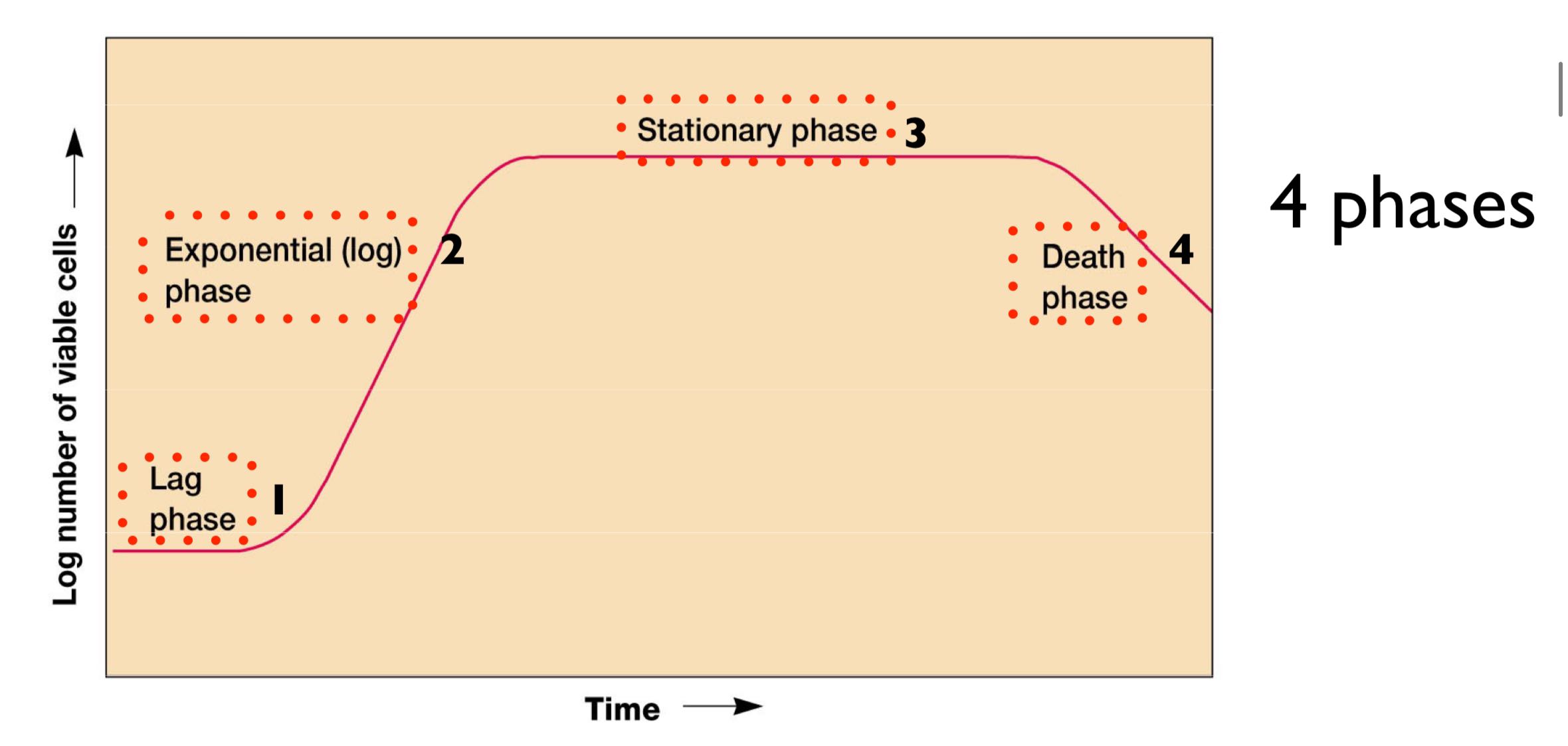
what are the 4 phases of the growth curve in a close system batch culture? where would penicillin be best adminstered?
lag phase (adjust environ./slow)
exponential phase (rapid/shortest gen time)
stationary phase (slowing growth/↓ nutrient)
death phase
exponential/log phase
what are sites of protein synthesis? what are the 2 subunits? what is the difference btwn bacteria/euk rRNA? what are the 2 subunits composed of?
bacterial ribosomes
2 subunits of protein + rRNA
70s ribosome: bacteria rRNA smaller/less dense than euk
30s small subunit = 16s rRNA
50s large subunit = 23S 5S
macrolides, chloramphenicol, aminoglycosides/tetracyclines —| ribosome function
what subunits are the 70S and 80S euk ribosomes made of?
70S = 50+30
80S = 60+40
what are the 3 types of antibiotics that target bacterial ribosomes?
macrolides: —| peptide transfer (bind 50S)
block elongation (x work Gram(-))
chloramphenicol: —| peptide bond (23S rNA)
cause aplastic anemia: ↓ RBC/leukocytes → bone destruction
aminoglycosides/tetracyclines —| tRNA bind w mRNA (bind 16s rRNA)
broad spectrum; bind Ca2+/teeth color
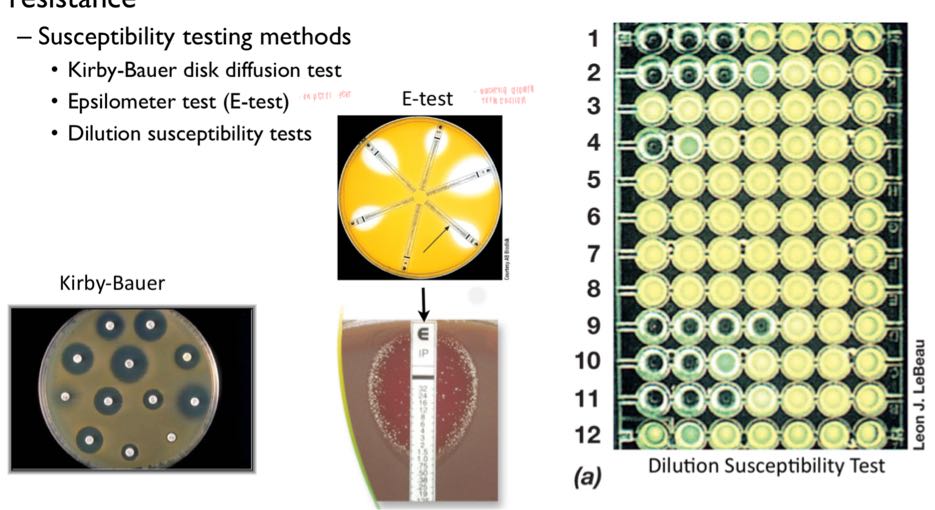
what are the 3 methods to measure antibiotic susceptibility?
kirby-bauer disk diffusion
largest diameter of growth inhibition
x tell bacteriostatic/cidal; shows MIC
epsilometer test (e-test)
dilution susceptibility test
lowest conc browth wo growth = MIC
that tube transfered n cultivated to test bacteriostatic/cidal
not info on MLC
how do antibiotics at baby affect microbiome?
↑ anitbiotics: ↓ diversity: ↑ allergies/disease
human microbiota x recover from antibiotic treatment
what are 4 ways bacteria develop resistance?
enzymes modify drug → destory/inactivate
alter binding target of drug
prevent drug entry
pump drug out
waht are the differences btwn viruses and bacteriophages?
viruses:
protein coat/capsid → dif shapes
classfification on nucleic acid present
contain DNA or RNA
enclosed by evelope (lipid) + spikes
infect specific cell type in one host
bateriophages:
DNA or RNA
life cycle regulation (repressor/activator)
lysis (lysins, holins: hole for phage escape)
x envelope virsus, x enter cell (only inject DNA/RNA)
well cahractertized genes
waht 5 things contribute to antibiotic resistance?
long term us eof single antibiotic
Widespread use
intact antibiotics in environ
clinical dosage > MIC
dif tissue/organ have ↑ inhibitatory conc
HGT (?) + anti.res. = naturally occuring
why was antibiotic resisitance found in the cave>
mutations = random
cave: ↓ resources: ↑ microbiome competition
antibiotics: ↑ survival
howw can fight antibiotic resistance? waht study with mice and what bacteria dound this?
fecal transplantation: restore normal gut microbiome (efficient probiotics)
(relman) c.difficile infected mice treated w cultured species from curative pssg → cured
who discovered bacteriophages?
felix d’herelle + frederick twort
bacteriophages found had bactericidal effect
d’herelle use phage cure cholera
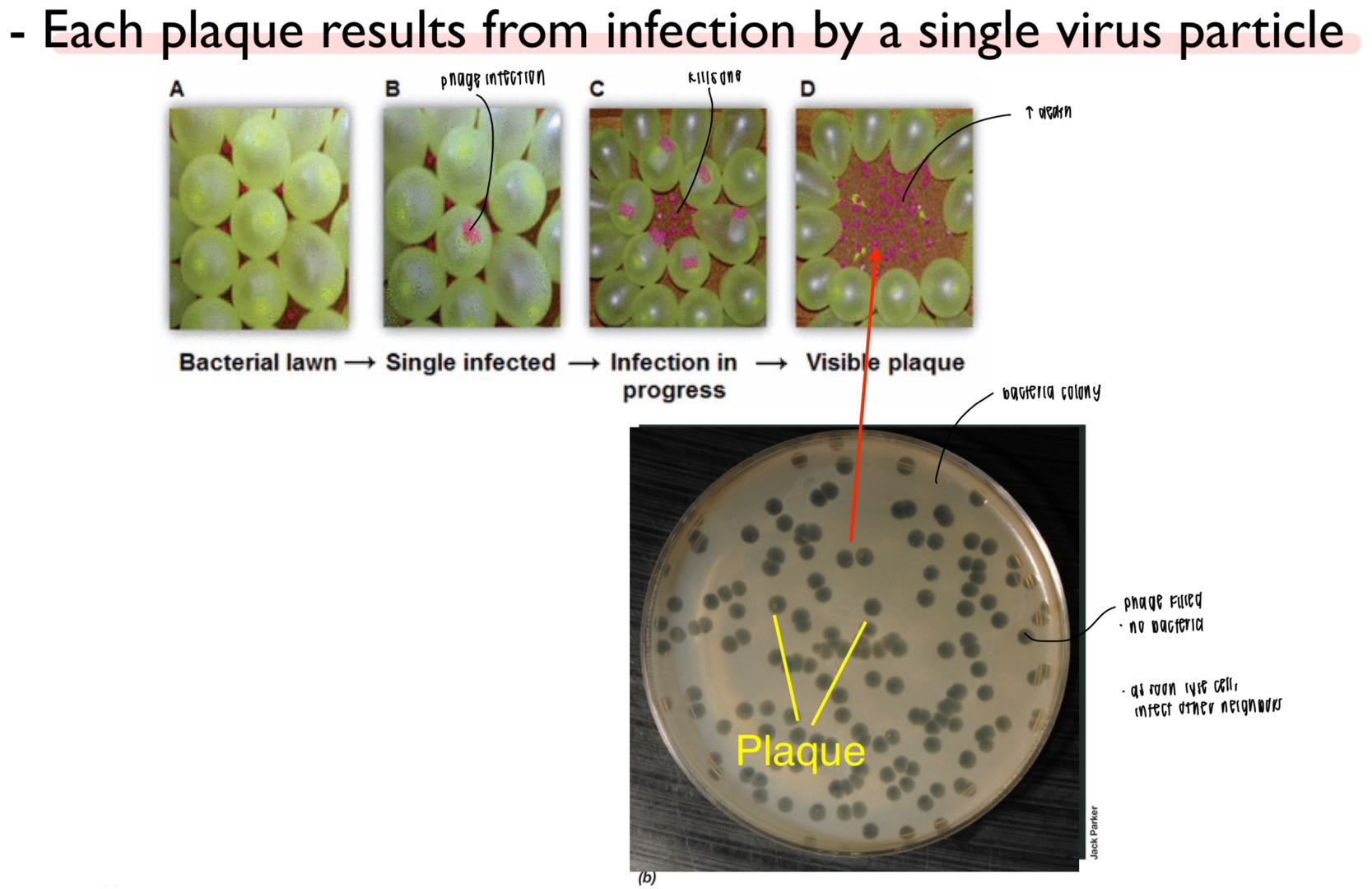
what was the plaque assay (d’herelle)?
measure virus infectivity; similar to disk diffusion
each plaque = infection from single virus particle
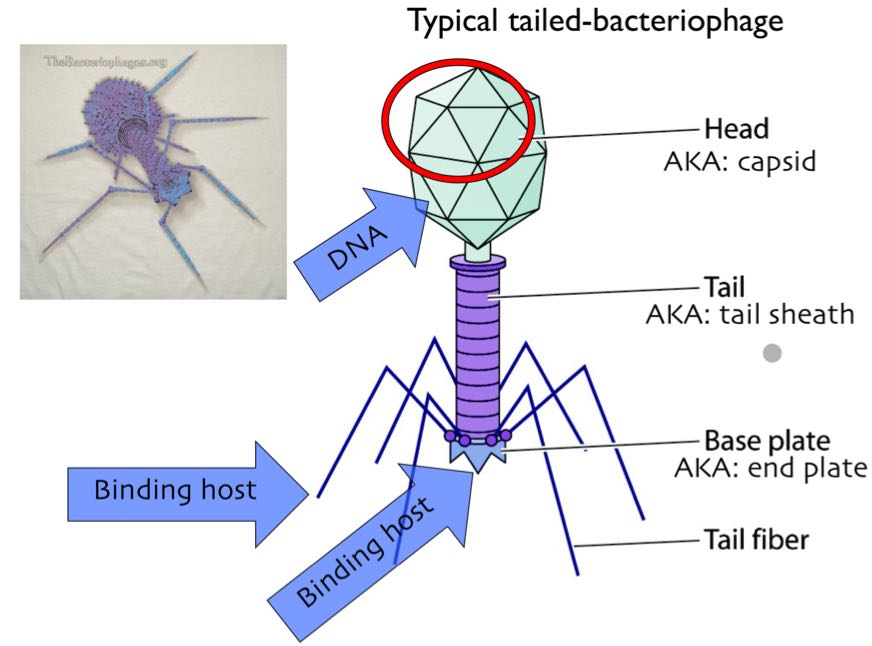
waht are the 4 types of bacteriophages? can they penetrate Gram(+)/(-) bacteria?
caudovirales (tailed)
siphoviridae (long, noncntractile tails)
myoviridae (contractil tail w sheath/cen tube)
podoviridae (short, noncontraile tails)
flagellum/tail fiber allow to bind + inject DNA
penetrate both Gram (+)/(-)
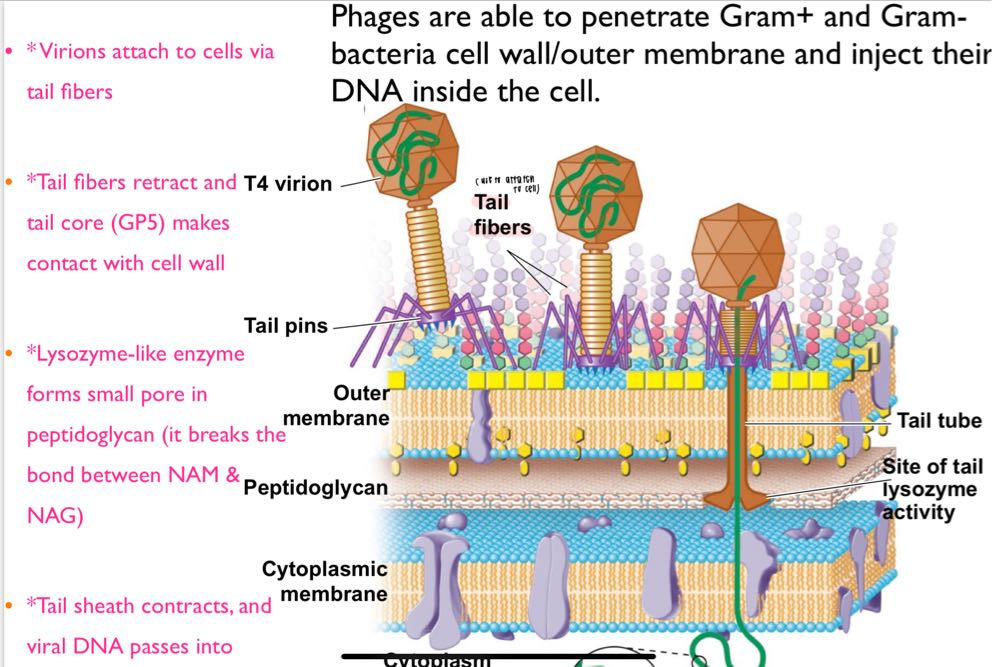
waht are the 4 steps a bacteriophage can penetrate gram(+)/(-)?
attatch to cell (tail fiber)
fiber retract + tail core (GP5) contact w cell wall
lysozyme-like enzyme froms small pore in peptidoglycan
tail sheath contrails + viral DNA enter cytoplasm
what 2 types of bacteriophages are there?
lytic phage: kills cell (more common)
temperate phage: lysogeny
parasitism/no viral reproduction/no death
benefits of phage therapy over anitbiotics?
phages jsut kill one species/strain
waht are teh physical/chemical requirements for growth?
physical:
temperature, pH, osmotic pressure
chemcial requirements
carbon, nitrogen, sulfur, phosphorous
trace elements
oxygen
waht is the use of chemicals for growth? waht 2 types of chemicals? what are the organisms called when they use those?
chemotrophy
organic chemicals (glucose, acetate)
chemo-organo-trophs
inorganic chemicals (H2, H2S, Fe2+, NH4+)
chemo-litho-trophs
waht is the use of light for growth? waht are those organisms called?
phototrophy
phototrophs
how did primirive cells metabolize on the early life on earth? where did oxygen come from
earth = anoxic (x oxygen)
anaeorbic metabolism; prolly chemolithotrophic
co2 → carbon | H2 → energy
primitive ATPase = proton motive force
2.7 bya cyanobacteria emerged O2 production
4.2 bya: great oxidation event (↑ O2 levels)
oxygen became best e- acceptor
what does the krebs cycle produce? what is it considered? what are the products?
½ glucose → 2 CO2 + 3 NADH + 1 FADH2 + 1 GTP
amphibolic: includes both catabolism/anabolism
how many ATPs are produces per 1 glucose in eukaryotes?
1 glucose → 38 ATP
1 NADH: 3 ATPs
FADH2: 2 ATPs
where is the ETC located in eukaryotes? bacteria?
eukaryotes: mitochondria
bacteria: plasma membrane
protons pumped across the membrane in the ETC allow for what 3 things? (proton motor force)
to produce ATP
to spin flagella (movement); w MOT protein
assist nutrient transport
how does the ATP synthase work as the protons move? what reaction occurs? is this reversible?
enzyme used to make ATP
as protons move in, gamma + epsilon subunits rotate → change active site
Pi + ADP → ATP (ccw)
this process is reversible*
*fermetative bacteria ATPase can only move one direction (ie. clostridium difficile)
in anaerobic respiration, what is the final electron acceptor in the ETC? how much energy does it yeild?
not oxygen; prolly nitrate, sulfate, carbonate
anaerobic < aerobic
in the eukaryote ETC, what are great electron donors? electron acceptor?
e- donor: NAD+/NADH
e- acceptor: oxygen
is the intestine strictly anaerobic? what keeps oxygen low in the intestinal lumen?
gastrointestinal tract mucosa (intestinal villi): capillaries: ↑ oxygen than intestinal lumen
facultative anaerobes choose to use oxygen so obligate anaerobes can survive
what 2 main bacterial phyla dominate gut microbiome?
firmicutes
bacteriodetes
how does immigration associate with gut microbiome?
↑ length US immigration: ↓ diverse gut microbiome: ↑ bacteroides: ↓ prevotella
bacteroides: sugar food breakdown
prevotella: plant breakdown