Chemistry - Unit 4: Bonding
5.0(1)
Card Sorting
1/32
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
1
New cards
so this whole unit is on bonding, but first, let's remind ourselves, WHY do atoms form bonds?
toooo beeee ~sttabbbbleeee~
remember that.
remember that.
2
New cards
what are ionic compounds typically composed of?
a metal (+) and a non-metal (-)
3
New cards
hey google, define "monatomic atom"
a single atom (key word: mono) with a + or - charge cuz of the loss or gain of electrons
4
New cards
in what order do the ions go when writing an ionic bond's name or formula?
cation (+) then anion (-)
5
New cards
what kind of structure do ionic compounds have?
a crystalline structure with alternating + and - charges
6
New cards
what are some characteristics of ionic bonds?
- transfer of electrons
- high melting points
- stronger bonds
- hard, brittle
- electrically reactive
- soluble in water
- high melting points
- stronger bonds
- hard, brittle
- electrically reactive
- soluble in water
7
New cards
why are ionic bonds soluble in water?
the alternating + and - charges allow for the water to easily break apart the particles
8
New cards
what kind of elements are found in covalent bonds
two or more non-metals
9
New cards
what are some characteristics of covalent bonds?
- sharing of electrons
- low melting points
- weaker bonds
- relatively soft (wtv that means)
- do not react to electricity
- not soluble
- low melting points
- weaker bonds
- relatively soft (wtv that means)
- do not react to electricity
- not soluble
10
New cards
what is another term for when two or more non-metals form a covalent bond?
it is considered a ~molecule~
11
New cards
we didn't learn a whole lot about this, but ur gonna need to know it. so what is a metallic bond?
the bond between metals, also considered the "sea of electrons"
12
New cards
describe the structure of a metallic bond pretty pls
crystalline structure, but electrons are mobile and allowed to run wild in the metal
13
New cards
what is the term for when electrons flow freely in metallic bonds
we call it -~zap zap electricity~-
14
New cards
pop quiz:
ionic vs. covalent vs. metallic bond. GO!
ionic vs. covalent vs. metallic bond. GO!
ionic bonds -> metal and nonmetal, electrons are GIVEN, totally transferred
covalent bonds -> 2 or more nonmetals, electrons are SHARED
metallic bond -> 2 or more metals, sea of electrons do whatever the heck they want
covalent bonds -> 2 or more nonmetals, electrons are SHARED
metallic bond -> 2 or more metals, sea of electrons do whatever the heck they want
15
New cards
wuts an alloy?
mixtures of 2 or more elements, BUT at least 1 must be a metal
ie: Brass -> Cu+Zn
ie: Brass -> Cu+Zn
16
New cards
how many electrons are found in a single bond?
2
17
New cards
how many electrons are found in a double bond?
4 (see the pattern? :D)
18
New cards
how many electrons are found in a triple bond?
6, you get the gist.
19
New cards
NAMING TIME! LESSGO
(don't flip the card over)
(don't flip the card over)
you little rebel.
20
New cards
this digital flashcard is tricky to work with, so here's what's gonna go down ->
I'll give you the name of a compound, and u determine the kind of compound (ionic or covalent) write the formula
OR
I'll give you 2 elements, u determine the kind of compound, name it, and write the formula :D
OR
I'll give you 2 elements, u determine the kind of compound, name it, and write the formula :D
21
New cards
Potassium + Sulfur
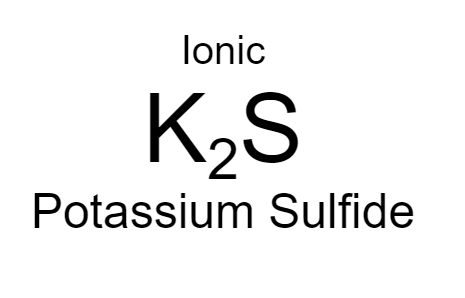
22
New cards
Dinitrogen Trisulfide
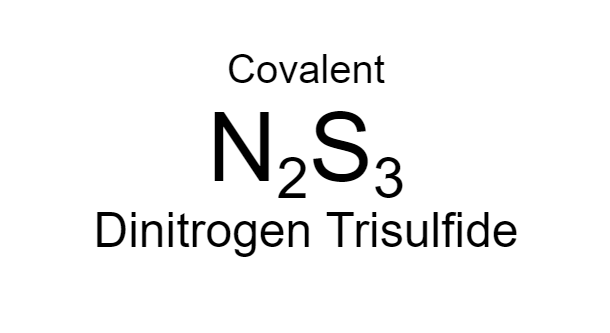
23
New cards
Copper (II) Phosphate
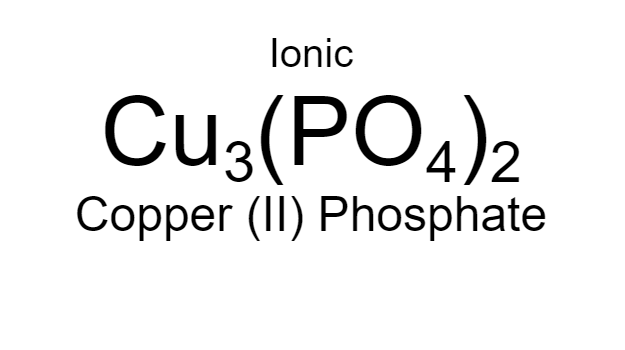
24
New cards
Cobalt (II) + Nitrogen
note: don't confuse the nitride, nitrite, nitrate. it's not fun.
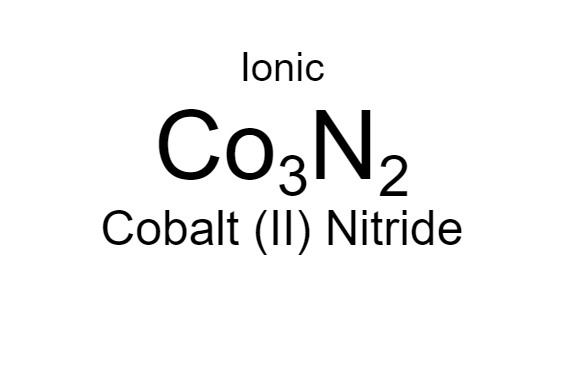
25
New cards
Carbon + Oxygen

26
New cards
time for...VSEPR
ok but I keep reading it as "VESPER" and then I read it as "VECTOR" as in
"VECTOR! That's me, because I commit crimes with both >DIRECTION< AND ~MaGNItuDE~. Oh yeAH"
carry on.
"VECTOR! That's me, because I commit crimes with both >DIRECTION< AND ~MaGNItuDE~. Oh yeAH"
carry on.
27
New cards
gimme the tea on Linear Bonds
- bonding pairs: 2
- lone electron pairs: 0
- lone electron pairs: 0
28
New cards
okay how about linear bonds' older sibling Linear w/ Multiple Lone Pairs?
- bonding pairs: 2
- lone pairs: 3
- lone pairs: 3
29
New cards
2 atoms will ALWAYS have a bond shape of...
linnnnearrrrr
haha I read that as "cleooouuurrrrr"
haha I read that as "cleooouuurrrrr"
30
New cards
describe the /bent\ bond
- bonding pairs: 2
- lone pairs: 1 or 2
- lone pairs: 1 or 2
31
New cards
how bout that Trigonal Planar bond?
- bonding pairs: 3 (like the sides of a triannngleee how clevah)
- lone pairs: 0
- lone pairs: 0
32
New cards
TRIGONAL PYRAMIDAL hehe that rhymes
- bonding pairs: 3
- lone pairs: 1
- lone pairs: 1
33
New cards
TETRAHEDRAL
sounds so pretentious
sounds so pretentious
- bonding pairs: 4
- lone pairs: 0
- lone pairs: 0