Lecture 2- Drug Discovery: From target Identification to Lead Optimization
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Project Initiation
Drug companies target markets, not disease:
Each new drug now costs more than $1 billion to develop
Companies must sell enough drug to recover R&D costs and achieve profitability
Market analysis
Competitive assessment
Research Analysis
Market Analysis
Number of potential customers
Type of potential customers
Can they pay for the drug
Nature of disease
Life-threatening, quality of life, lifestyle
Acute(short-term) vs chronic (Long term)
Current treatment
Cost
Effectives
Safety
Convenience
Competitive assessment
What are competitors doing?
TIming of market entry and profitability:
1st drug to market makes the most revenue
2nd drug makes less overall revenue but often still profitable due to lower development costs
3rd drug may only achieve limited profitability
4th and beyond have difficult recovering costs (R&D)
What are the advantages/disadvantages?
Safety and efficacy for existing therapies
Cost and accessibility
Convenience and patient adherence
Feasibility Study (Biochemistry)
Focus on solvable problems
Is the disease well understood?
Clear diagnosis
Can it realistically be treated with drugs?
Is the mechanism of disease understood?
Black box diseases are difficult to target
Are biochemical tools available?
Reliable assays to test drug activity
Suitable animal models
Are there existing drugs?
Potential starting point for research
Proof of principle that the approach can work
Competition or patent barriers
Biochemical studies
Proof of principle
Does the idea have a chance of working?
Is there experimental evidence supporting the concept?
Drug testing methods
Reliable ways to test candidate drugs
Often multiple methods are developed (fast, accurate, biologically relevant)
Development of animal models
Create or exaggerate disease state to study drug effect
Medicinal chemistry starts with a lead
Show activity in the test being used
Some specificity
Not active in unrelated tests
Pattern of “drug-like” properties
Must be able to enter and function in the body
Patent opportunity
Chemistry must be feasible
Structure can be modified
Practical and efficient synthetic routes
Lead Identification
Medical chemistry requires a starting point
Lead
Methods used to identify leads
High throughput screening (HTS)
Natural products
Rational drug design
Combinatorial chemistry
De-novo design
High-Throughput Screening (HTS)
Thousands of compounds are tested for activity
Typical libraries contain >500,000 compounds
Companies maintain large compound collections, including:
Every compound that was ever made in the company
Compounds purchased from academic labs
Natural products
Requires a biological assay that can be automated
Modern robotic systems can screen >200,00 compounds per week
Compounds showing activity (hits) are tested more carefully’
Importance of testing a Variety of Compounds
No way to predict which compounds will work in advance
Drugs and biological molecules interact in many unpredictable ways
Testing only small variations of the same compound limits discovery
A large variety of chemical structures and types increases the chance of finding hits
HTS Testing
All compounds are tested at the same dose
Typical concentration: ~30 nanometers
Compounds tested one at a time
Clear individual results
Biological assay provides a simple yes/no answer
Counter-screens help remove false positives
Assay with related biological functions
Desired compounds are specific (active only in the main assay)
Good HTS generates ~500 hits
Hit = compounds that tests positive in HTS
Hit- to Lead Removes False positives
Most initial hits are false (>99%)
Impurities
Decomposition products
Compound reactivity
Detergent-like behaviour
Redox reactions
Strong electrophiles or nucleophiles
Interference with assay
Hydrophobic compounds(stick to protein or plastic)
Assay artifacts (colour, fluorescence)
Poor solubility
Goal: eliminate false hits quickly
Pan-Assay Interference Compounds (PAINS)
Promiscuous bioactive compounds
Show activity in almost any biological test
Certain chemical structures react nonspecifically with biological targets ( some interfere with test methods)
Redox activity
Detergent-like properties
Strong acid/base behaviour
Strong nucleophile/electrophole interactions
Photoreactivity
Chelating properties
High lipophilicity
Re-testing hits
Re-test compounds using purified samples
Test at multiple concentrations
Compounds act in concentration-dependent manner
Dose response curve
Test in other Assays
Test in available assays
Previously collected data for the compounds is re-examined
Reject promiscuous compounds (PAINS)
Structure confirmation
Spectroscopy and Independent Synthesis
Several compounds in collections are very old
Decomposition (during storage)
Incorrectly identified
Synthesize, purify, confirm structure and re-evaluate
Test a series of compounds with related structure
Compounds in the series should all be active, but show difference that can be related to structural patterns
Hit-to-Lead Required 3 to 6 Months
Drug developed by HTS
Nevirapine
Gleevec
Quinolones
All contain Aromatic rings
Natural products as Leads
Chemicals isolated from plants, animals, microbes
Secondary metabolites
Chemical not directly required for life
Produced by the organism for a secondary purpose
Poisons for defense or against predation
Colors, fragrances
Hormones, pheromones
Modify the organism's environment
Searching for natural products
ollect a large amount of a source organism
Plant, animal, microorganisms
Isolate and purify substance showing biological activity
Determine the chemical structure
Confirm biological activity
May use information about the source organism to narrow search
Poisonous organism
Local knowledge
Natural product leads
Isolate from living things
May require a large amount of raw material (tons)
Often difficult to perform SAR
Complex structures
Limited to chemical modification of the natural product
Academic labs only
Companies closed NP departments in 1980s
Natural product structures
Penicillin
Taxol
Digoxin
Drugs produced in Industrial quantities
More than 20,000 tons of penicillin per year
Must be farmable or produced by industrially compatible method
Spongistain
400kg of marine sponge provided only 13.8g of spongistain
Found to be one of the most potent tumor-growth inhibitors ever discovered
Attempt to re-isolate the compound unsuccessful
13 tons of wet sponge gave just 35 mg
The sponge was extinct by the 3rd attempt
Rational drug design
Design a lead using a known chemical structure
Enzyme substrate
Natural inhibitor
Ligand for a biological receptor
Existing drug
Knowledge of the mechanism of action
Design for improved properties
Captopril Resembles snake venom
Acycolvir resembles guanine
Rationally Designed drugs
Captopril
Acyclovir
Indinavir
Combinatorial Chemistry
Semi-random synthesis of a large library of related compounds
Robotic synthesis
Text mixtures (academic)
Test impure single product (industry)
Based on the idea of Darwinian selection
Combinatorial search
Make a large mixture (library of molecules)
Structures have a common “core) and random “appendages)
Made using similar chemical reactions
Test the compounds as a mixture
“Deconvoulte” the result from the mixture to identify molecules that show desired properties
One drug discovered this way
Sofrafenib
Kidney and liver cancer (rare)
Combinatorial chemistry used to identify an alternative core “lead” after hit-to-lead
De-novo design
Use a computer to design a lead from scratch
Required the 3D structure of biological target
X-ray crystal structure
NMR structure
De-novo Drug Design
Start with a protein structure
Build a computer model of the binding site
Design a molecule that fits into this pocket
Lead optimization
nce a lead is available, optimization begins
Focus on medical chemistry
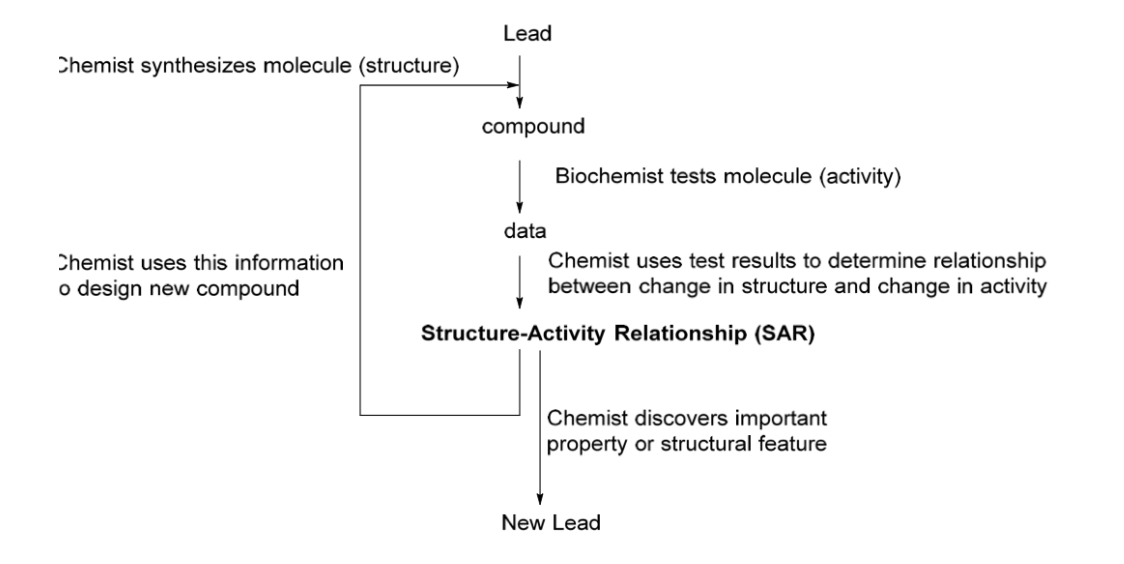
Structure Activity Relationships (SAR)
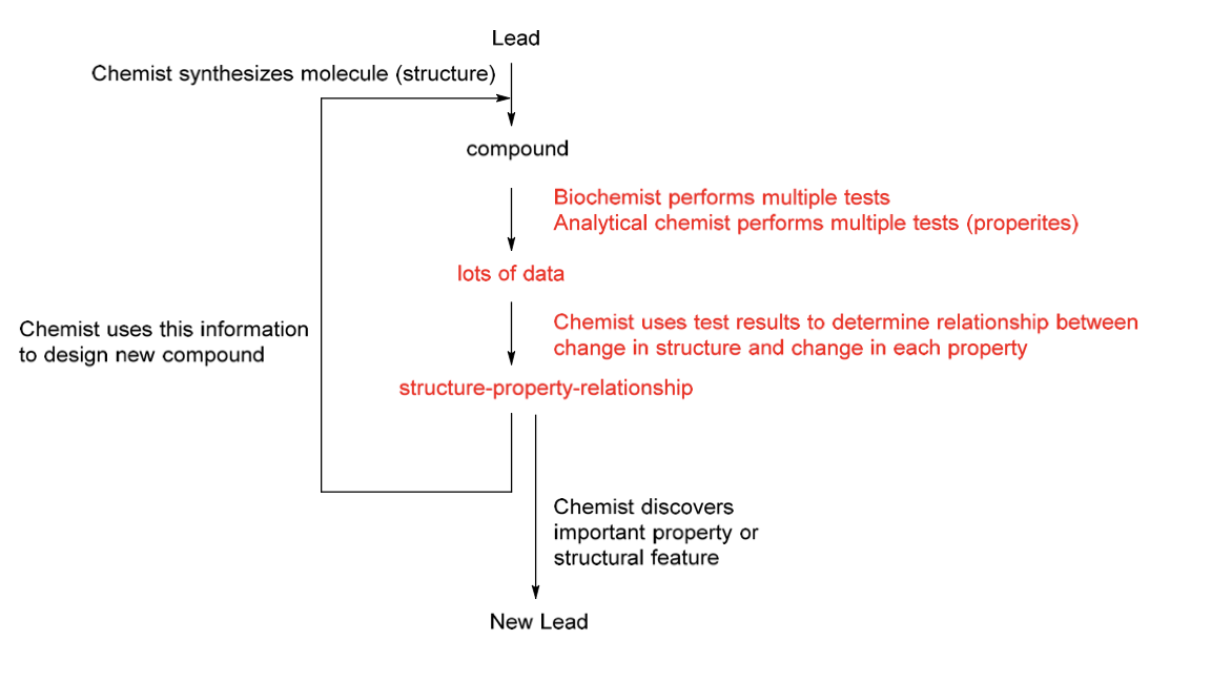
Structure properties Relationships (SPR)
Make small changes to a drug's structure and observe changes in activity
Identify parts of the molecule that interact with biological target
Optimize as many properties as possible
Molecules made one at a time (SAR)
Chemist designs new molecules based on lead structure
Chemistry synthesizes the designed molecule (structure)
Biochemist tests the synthesized molecule (activity)
Chemistry analyzes the results to determine the relationship between structural modifications and changes in activity
Chemist uses this information to design new compounds
SAR as a process
Structure Property Relationships (SPR)
Optimize several properties simultaneously
Potency
Dose that produces biological activity
Aim for the lowest dose possible (low dose = high potency)
Selectivity
Ratio of dose that gives desired biological activity vs undesired activity
Aim for the highest possible dose for undesired biological activity (high dose = high selectivity)
Solubility (in water)
Lipophilicity (solubility in non-polar solvents)
Chemical stability (time and conditions for decomposition)
Acid-base behaviour
Susceptibility to metabolism (rate and type)
Toxicity (nature and dose)
Ease of synthesis (speed and efficiency)
SPR as a process
Focuses on molecules that can enter the body
One of the hardest problem in medical chemistry is getting drugs into the body
Avoid wasting effort on compounds that can never become drugs
Focus on “drug-like” molecules
Certain properties correlate well with drug performance, optimize these simultaneously with potency
Requires extensive computational support
Drug Candidate
End result of the discovery phase
Chemical properties are known
Basic biological profile is known
Process required 1 to 3 years
Approx 2000 to 5000 chemical compounds are made and tested to find each candidate
Companies often identify 3 or 4 backup compounds
If the primary compounds, minimize recovery time