Unit 3 IB CHEM
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
Chemical Bonding
Atoms bond to achieve stability.
A chemical bond holds chemical compounds together
Three types of bonding:
Ionic: between a metal & a nonmetal or polyatomic
Covalent: between two or more nonmetals
Metallic: between metals - alloys
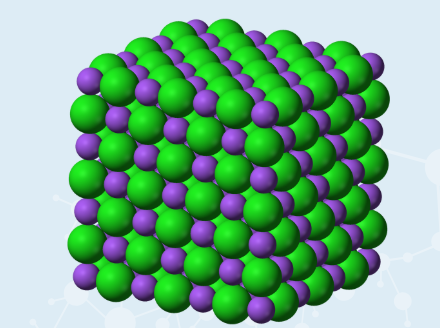
Ionic Structure
Ionic Compound form a large crystal lattice
no individual molecules
Covalent Structure
Covalent compounds form individual molecules
Covalent Compound properties
Solids are usually soft
Ex: Wax
Typically insoluble in water
Do NOT conduct electricity
Melting points and boiling points are usually low
Covalent Properties (continued)
Between nonmetals
Electrons are shared
Equal sharing causes non-polar bonds
Example: H2
Unequal sharing causes polar bonds
Common Substances
Ammonia - NH3
Carbon Monoxide - CO
Carbon Dioxide - CO2
Water - H2O
Solid (s) State of Matter
Fixed volume and shape
Particles close together and vibrating in position
Liquid (l) State of Matter
Fixed volume, variable shape
Takes the shape of its container
Particles are packed moderately-tightly together
Gas (g) State of Matter
Variable volume and shape
Particles far apart and moving very fast
Aqueous (aq) State of Matter
Fixed volume based on the solution
Variable shape
Particles evenly distributed throughout water
Ionic compounds dissociate (separate into ions and conduct electricity)
Nonpolar covalent bond
electrons are shared equally
Electronegativity difference between 2 bonded atoms = 0.0-0.4
Polar covalent bond
electrons shared unequally (partial charges)
Electronegativity difference between 2 bonded atoms =0.5-2.1
Ionic Bond
A bond between a metal and a nonmetal
Electrons are transferred (full charges)
Electronegativity difference between 2 bonded atoms = 2.2 - 4.0
Trigonal Planer Bond Angle
120' degrees
Linear Bond Angle
180’ Degrees
Tetrahedral Bond Angle
109.5’ degrees
Trigonal Pyramid Bond Angle
<109.5’ Degrees
Bent VSEPR bond angle
<109.5’ Degrees
Molecular polarity
For a molecule to be polar it must have a δ+ and a δ- side.
The molecule must have a non-symmetrical distribution of electrons.
Molecular polarity (how to tell)
Does the molecule have a lone pair of e- on the central atom?
Yes – the molecule is polar
No – go to the second question
Are any of the atoms bonded to the central atom different?
Yes – the molecule is polar (different atoms are bonded to the central atom)
No – the molecule is nonpolar (all the atoms bonded to the central atom are the same)
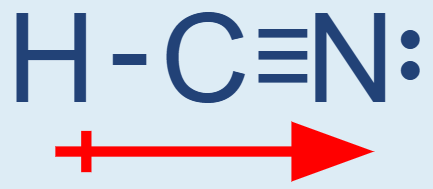
Molecular Polarity Arrow
To indicate direction of polarity draw an arrow with an extra line – the + is on the H (low EN) and the head goes toward the element pulling the e- (N – high EN)
Diatomic Molecules
Br2, I2, N2, Cl2, H2, O2, F2
Come in pairs
More stable this way
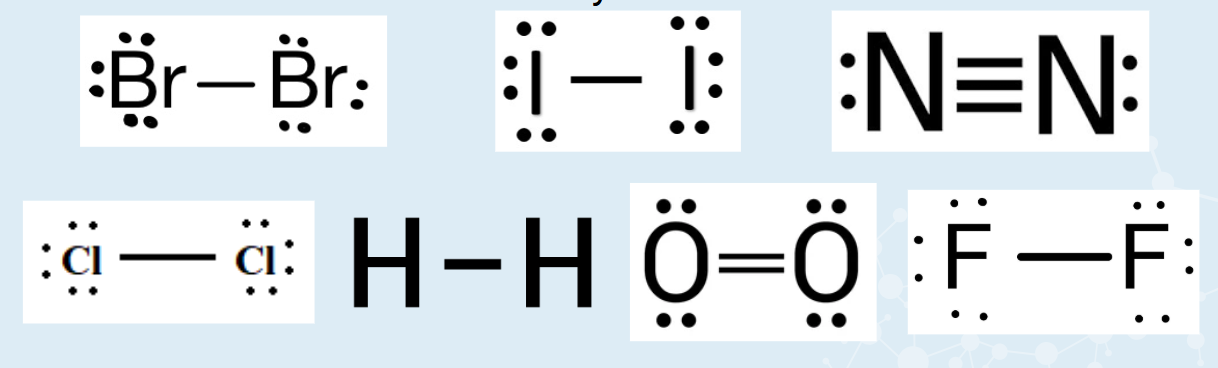
Saturated Hydrocarbons
Saturated
no double or triple bonds
Hydrocarbons
containing hydrogen and carbon
Are Alkanes
generally inert (non-reactive)
Due to strength and stability of C-C (348 kJ mol -1) and C-H (412 kJ mol-1) bonds – bond enthalpy
Non polar
Straight chain alkanes
Single bond between C-C
1 Carbon
Methane
CH4
Different Representations of Chains
Molecular Formula
C3H8
Condensed Formula
CH3CH2CH3
Expanded Structural Formula
Organic Structure
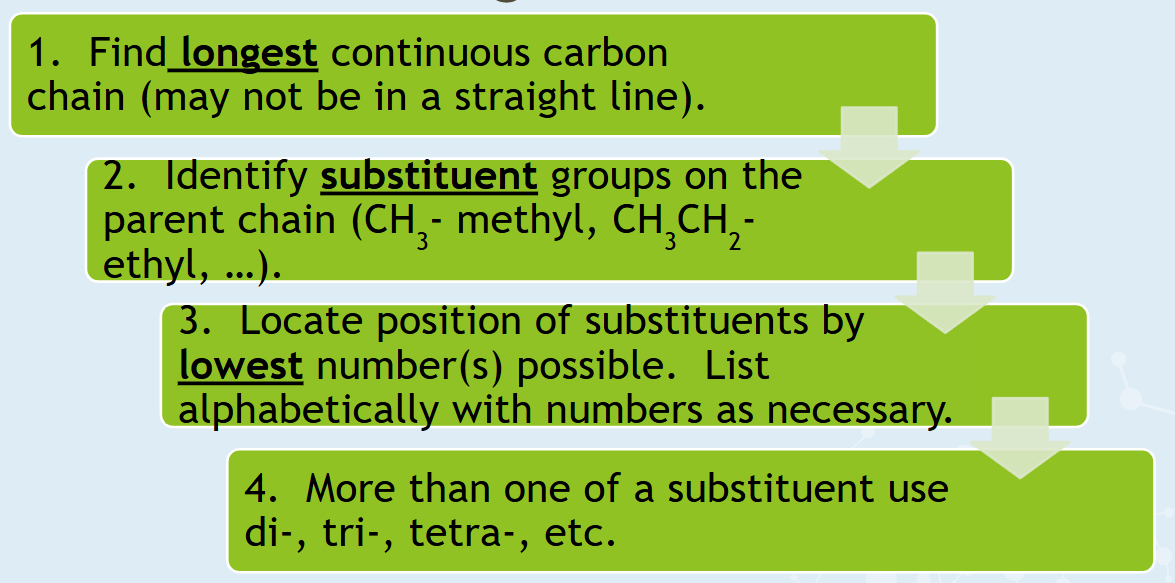
Rules for naming branched alkanes...
Unsaturated Hydrocarbons
Unsaturated
containing a double or triple bond
C=C Double bond - Alkenes
Formula: CnH2n
C≡C Triple Bond - Alkynes
Formula: CnH2n-2
Isomers
compounds with the same molecular formula but different structural formulas
Naming alkenes with substituents

Properties of Hydrocarbons
Intermolecular Forces (IMF’s)
Nonpolar molecules
Only C-H and C-C bonds
Intermolecular Force
London- Dispersion (Van der Waals Forces)
Increases with mass
Decreases with branching
Properties of Hydrocarbons (extended)
Homologous Series
Differ by additional -CH2-
Physical Properties:
Melting Points/Boiling Points will increase with mass (number of carbons)
Chemical Properties:
Similar
Due to similar types of bonds
INTERmolecular
Attractive forces between a group of molecules
Weaker than covalent bonds
Can be broken to change state of matter without changing formula of a compound
INTRAmolecular
Forces within a molecule
Strong covalent bonds
Very strong compared to IMFs
Types of Intermolecular Forces
London Dispersion Force (LDF)
Dipole - Dipole
Hydrodgen Bonding
London Dispersion Force (LDF)
London dispersion forces (LDF)
Occurs between ALL molecules (polar AND nonpolar)
Have low melting & boiling points
instantaneous temporary dipole
larger molecules have greater attractions
no partial charges
Dipole Dipole
Dipole forces (dipole-induced dipole)
Stronger than LDF
Attraction between permanent polar molecules
Partial charges attract each other
Hydrogen Bonding
Strongest Intermolecular Forces
Special polar molecules
Form when Hydrogen is bonded to either:
Fluorine
Oxygen
Nitrogen
Which makes Hydrogen bonding FON
Why H-bonds are the strongest
The strength of the Hydrogen bond is due to the electronegativity difference between hydrogen on one molecule and F, O, or N on a different molecule.
Remember, F, O, and N are the most electronegative on the periodic table.
Which Molecules have what IMF?
All Molecules have LDF
Polar Molecules have both LDF and Dipole - Dipole
Molecules that have FON bonded with a H have H-bonds as well as both other types
Bonds and forces
All chemical bonds are strong
Atom to atom - Nonpolar and polar covalent compounds (molecules)
Ion to ion – ionic compounds
Ionic Compounds have a network of bonds in a 3-D crystal lattice
Molecular compounds have weak attractions between the molecules called intermolecular forces
Macromolecular
Non-metal atoms that are covalently bonded into a lattice structure (e.g., diamond, sand)
Very rigid structure due to high number of shared electron pairs and covalent bonding throughout structure.
No IMFs because there are no small molecules
Molecular polarity
If the molecule is symmetrical, it is nonpolar
If the molecule is asymmetrical, it is polar
Lone pairs of electrons or different elements on the central atom typically make a molecule asymmetrical
IMFs and physical states
Stronger IMF’s make molecules more attracted to each other
Stronger attractions take more energy to break and change states of matter
Remember it can be combination of
Dispersion – mass
Dipole-dipole – partial charges
Hydrogen bonding – very large partial charges
Physical properties of IMFs
Melting/boiling point, volatility, conductivity and solubility of compounds can be predicted based on IMFs and bond type
Stronger IMF/attraction = higher MP/BP and lower volatility (evaporates less)
More free-moving electrons/ions = higher conductivity
More polar = higher solubility in water, lower solubility in non-polar solvents
Less polar = higher solubility in non-polar solvents, lower solubility in water
Physical properties of Covalent, Ionic, and metallic
| Covalent Molecules | Ionic Compounds | Metallic |
Hardness/ Malleability | Soft and malleable Often gasses | Hard and brittle | Hard and malleable |
MP/BP | Low (H2O MP 0ºC, BP 100ºC) | High (NaCl MP 801ºC BP 1465ºC ) | Higher (Fe MP 1538 ºC, BP 2862 ºC) |
Conductivity | None in all states | Good in (l) and (aq) states | Good in (s) and (l) states |
Solubility (will or will not dissolve in) | If polar, good in H2O If nonpolar, not good in H2O | Good in water | Only in other metals |
Examples | CH3OH CO2 (polar) (nonpolar) | NaCl CaCO3 | Fe, Au, Ag, Cu, Al, Pb,... |
Not a Flashcard
Physical properties of Covalent, Ionic, and metallic
| Covalent Molecules | Ionic Compounds | Metallic |
Hardness/ Malleability | Soft and malleable Often gasses | Hard and brittle | Hard and malleable |
MP/BP | Low (H2O MP 0ºC, BP 100ºC) | High (NaCl MP 801ºC BP 1465ºC ) | Higher (Fe MP 1538 ºC, BP 2862 ºC) |
Conductivity | None in all states | Good in (l) and (aq) states | Good in (s) and (l) states |
Solubility (will or will not dissolve in) | If polar, good in H2O If nonpolar, not good in H2O | Good in water | Only in other metals |
Examples | CH3OH CO2 (polar) (nonpolar) | NaCl CaCO3 | Fe, Au, Ag, Cu, Al, Pb,... |
Not a Flashcard