CHEM 1211 Section 7.4 (Limiting Reactants and Percent Yield)
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
run out
In most reactions, one reactant will ___ ___ before the others.
Limiting Reactant (Limiting Reagent)
the reactant that runs out first
Excess Reactant (Excess Reagent)
the reactant that doesn’t run out first
Theoretical Yield
The ideal amount of product that a reaction can make mathematically
Actual Yield
The amount the reaction produces in the laboratory
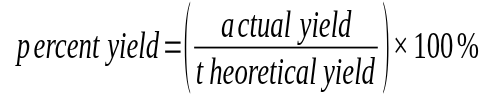
Percent Yield
The ratio of actual yield to percent yield

3.345 mol CO2
If you start with 6.71 mol O2, how many moles of CO2 will be produced?

6.71 mol CO2
If you start with 6.71 mol CH4, how many moles of CO2 will be produced?
ratio
To determine the amount of moles produced in a reaction, determine the _____ between the reactants and the product.

O2
__ is the limiting reactant because it produces half as many moles as CO2.
Reactant
a substance that is consumed in a reaction
Reagent
any substance mixed during a reaction, including catalysts, solvent and unconsumed indicators