4 Acids and Redox
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
formula of 4 common acids
HCl, H2SO4, HNO3, CH3COOH (ethanoic acid)
formula of 3 common alkalis
NaOH, KOH, NH3
what do acids do in aqueous solution
release H+ ions
what do alkalis do in aqueous solution
release OH- ions
explain strong and weak acids in terms of relative dissociations
strong acids fully dissociate
weak acids partially dissociate
ionic formula for neutralisation reaction
H+ (aq) + OH-(aq) --> H2O(l)
describe neutralisation
the reactions of acids with bases (including carbonates, metal oxides and alkalis (water-soluble bases), to form salts
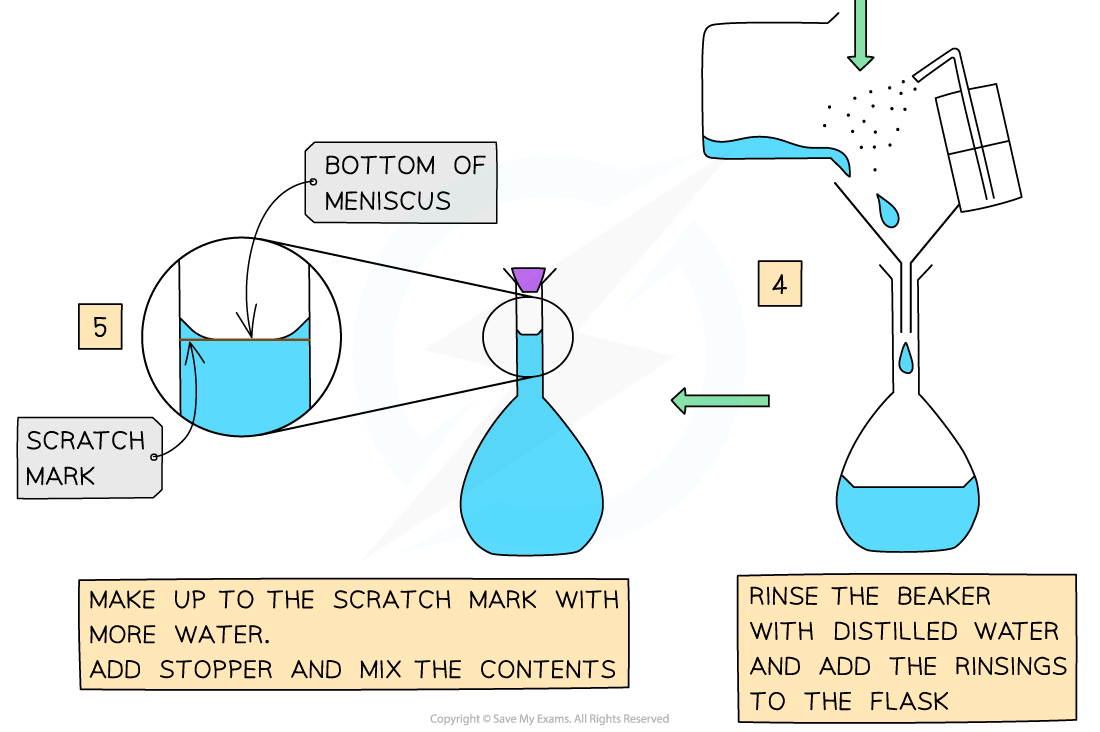
steps to prepare a standard solution
weigh a precise amount of solid
add solid to small volume of distilled water in a beaker and dissolve
add to a volumetric flask
rinse the beaker with distilled water and add the rinsings to the flask
make up to the bottom of the meniscus of the graduation line with more distilled water in volumetric flask
add stopper and invert flask a few times to mix contents
describe oxidation and reduction in terms of changes in oxidation number
increase in oxidation number = oxidation
decrease in oxidation number = reduction
disproportionation
a redox reaction in which the same element is both oxidised and reduced
acid def
a proton donor
gives off H+ ions when dissolved in water
base def
proton acceptor
a compound that neutralises an acid to form a salt
alkali
a soluble base
releases OH- in aq solutions
strong acid def
a proton donor that fully dissociates in water
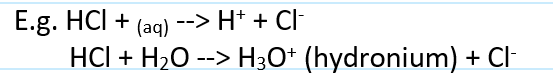
examples of strong acids
HCl, H2SO4, H3PO4 (kinda), HNO3
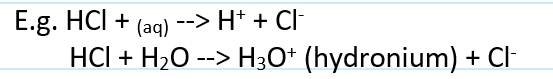
strong base def
a proton acceptor that fully dissociates in water
e.g. NaOH
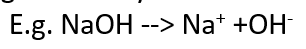
weak acid def
a proton donor that partially dissociates in water
what kind of acids are weak
all organic acids
e.g. HCOOH methanoic acid
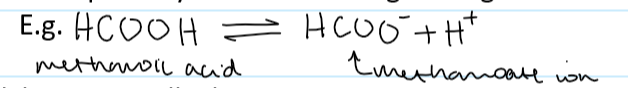
weak base def
a proton acceptor that only partially dissociates in water
weak base example
NH3

acidic oxide
non-metal oxides
can donate protons when in water
basic oxide
metal oxides
can accept protons when in water
amphoteric
can react w acids and bases
example of an amphoteric compound
aluminium oxide Al2O3
amphiprotic
can donate and accept protons
example of amphiprotic compound
water, aluminium hydroxide Al(OH)3
redox
a reaction where oxidation and reduction take place. a chemical element is both consumed and produced
weak acids/bases use what sign in their reaction
reversible reaction sign
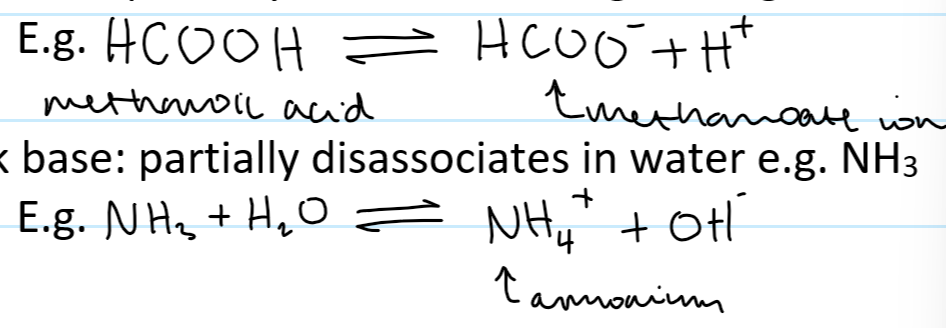
describe oxidation state
the charge on an element in a compound if all bonds to that element are 100% ionic
oxidation state rules
elements are always 0
in compounds:
O = -2
H = +1
Grp1 = +1
Grp2 and Zinc = +2
how to find out the overall charge of a compound
the sum of the oxidation states
is the sign before or after the number in oxidation numbers
before the sign
special cases of oxidation numbers
H in metal hydrides = -1
e.g. NaH
O in peroxides = -1
e.g. H2O2
O bonded to F = +2
e.g. F2O
modern name for nitrite NO2-
nitrate (III)
ox number of nitrogen in nitrite NO2-
+3
ox number of nitrogen in nitrate NO3-
+5
diprotic acid example
H2SO4
what kind of acids are good conductors of electricity and why
strong acids
more ions in aqueous as more H+ dissociate
concentration has no correlation to the strength of the acid
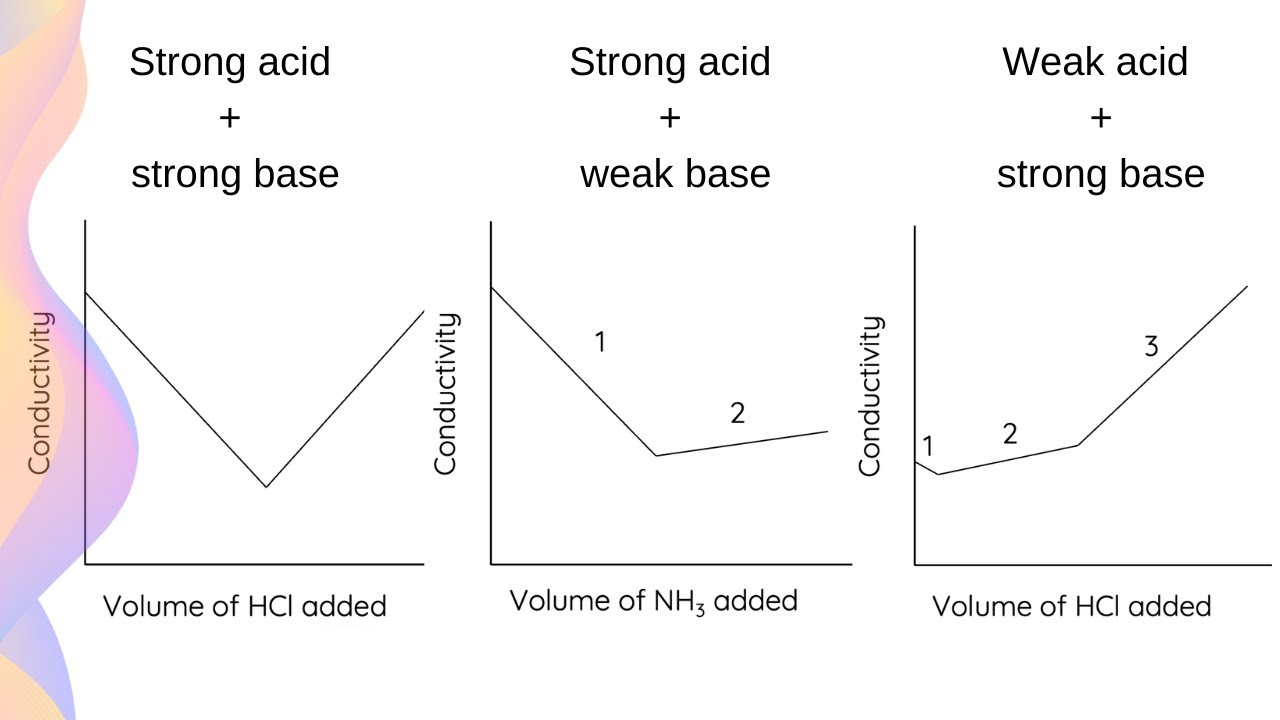
when can u do a conductimetric titration
when there are no ions on one side of the reaction
graph for conductimetric titration
formula for sulfate (VI)
SO42- sulfate
formula for sulfate III
SO32- sulfite
formula for nitrate V
NO3- nitrate
formula for nitrate III
NO2- nitrite
formula for chromate VI
CrO42-
formula for dichromate VI
Cr2O32-
wording for describing what has been oxidised/reduced by oxidation number
Cu in CuO has been reduced from +2 to 0 in Cu
include element and compounds
include oxidation number change
why is this reaction a neutralisation reaction
an acid has been neutralised by a base to form water
why is calcium nitrate a salt
the H+ ion in an acid has been replaced by a metal ion/Ca2+
what is a Cl with a ox state of 7 called
Chlorate VII