Biochem Sept. 11th
1/24
Earn XP
Description and Tags
(Topic 3)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Can a peptide bond rotate? Why?
No, peptide bonds cannot rotate due to the partial double bond character resulting from resonance. This rigidity maintains the protein structure (N-Cα and Cα-C’ bonds can rotate though).
Tell me about the Primary Structure of a protein
The linear sequence of amino acid residues in a protein.
Formed by peptide (amide) bonds
CAN’T be denatured by urea (urea can’t break peptide bonds)
*Just knowing the sequence alone can’t reveal the 3D structure
Whats a dipeptide?
A peptide chain composed of 2 amino acid residues
Whats a tripeptide?
a peptide chain composed of 3 amino acid residues.
Whats a oligopeptide?
A peptide chain composed of 2 to 20 amino acid residues.
Whats a polypeptide?
a peptide chain composed of many more residues
Whats a protein?
a molecule composed of one or more polypeptide chains
Tell me about the Secondary Structure of a protein
Local spatial arrangement of the backbone of the polypeptide
Two major types: alpha helices and beta sheets.
Stabilized by hydrogen bonds (between backbone N-H and C=O groups)
(Secondary structure explains how the backbones of the polypeptide interact through hydrogen bonds)
Since Glycine lacks a side chain, it’s highly flexible; can also disrupt secondary structures so less likely to find one in alpha helices and beta sheets
What are hydrogen bonds?
a bond between a hydrogen atom and an electronegative atom, such as oxygen or nitrogen, when the hydrogen itself is covalently bonded to another electronegative atom
Proline can’t participate in hydrogen bonding because its backbone nitrogen is part of a ring structure (doesn’t have that extra amide H)
When placed in a secondary structure, it often induces kinks or turns, affecting the overall protein conformation.
Tell me about the Alpha Helix Structure
Rigid cylinder structure
C=O of each residue forms a hydrogen bond with the backbone N-H group 4 residues ahead
Won’t normally find proline in alpha helices because it can’t participate in hydrogen bonding AND isn’t very flexible, which is important for alpha helix structure
Tell me about the Parallel Beta Sheet Structure
Connected by hydrogen bonds between backbone atoms of adjacent strands (angled, making them less stable then the antiparallel beta sheets)
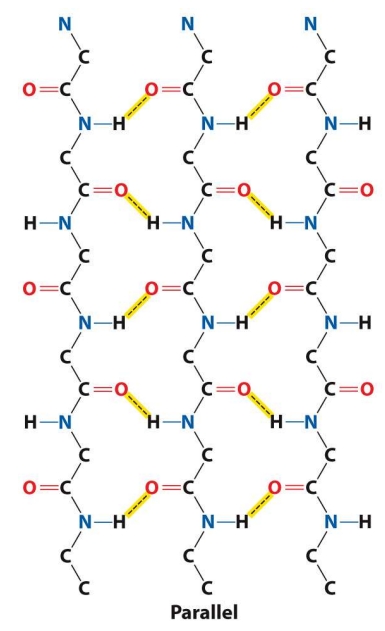
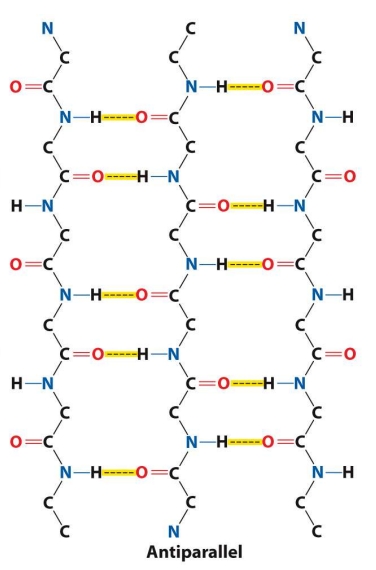
Tell me about antiparallel beta sheet Structure
Tend to be have more linear (stronger) hydrogen bonds because the donor, hydrogen, and acceptor lie on a straight line
What are the forces stabilizing protein structures? (aqueous environments)
Covalent bonds (strongest stabilizing interactions)
Ionic interactions between oppositely charged residues (can be strong but weakened in water)
Hydrogen bonds (contribute to stability, especially in secondary structures)
Van der Waals interactions contribute to tight packing of the hydrophobic core and overall stability
Why does the environment matter for bonding?
In aqueous solutions, ionic interactions are weaker than in vacuum because water stabilizes separated ions
Disulfide bonds only form in oxidizing environments because cysteine residues must lose electrons to bond
Reducing environments prevent their formation or break existing disulfide bonds, restoring -SH groups
The common charged residues?
Lysine and Arginine (+)
Glutamate and Aspartate (-)
Ionic interactions can occur between these two groups when close to each other
Tell me about the Tertiary Structure
The overall 3D arrangement of a single polypeptide chain, including how its side chains (R groups) interact with each other and with the backbone
Describes the compact folding of the entire chain into a unique 3D shape
*Can have alpha and beta sheets mixed together
Stabilized by interactions between R groups of amino acids
hydrophobic interactions
Hydrogen bonds
Ionic (electrostatic) interactions
Disulfide bridges
Tell me about the Quaternary Structure
Spatial arrangement of multiple polypeptide chains (subunits) in a protein that contains more than one chain
Subunits may assemble independently and then come together to form the complete protein
Like hemoglobin and all its subunits with its distinct behaviours
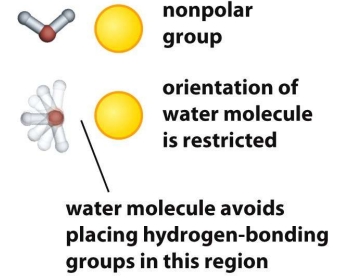
Tell me about the Hydrophobic Effect
Major driving force for protein folding: hydrophobic side chains are more likely to be found in the core of the protein
When the protein is unfolded, exposed hydrophobic groups interact unfavorably with water, causing water to reorganize around them in ordered configurations, reducing the entropy of the surrounding water (decreases disorder)
By burying hydrophobic residues inside the protein, water can form a larger number of favorable hydrogen bonds with itself, increasing the overall entropy of the system (despite the protein becoming more ordered). This net entropy gain for the system favors folding (ΔS increases = disorder increases)
Folding is favoured by Gibbs free energy because its a spontaneous reaction (G<0)
What do Chaperones do?
Chaperones help newly synthesized polypeptides fold into the correct conformation and prevent misfolding or aggregation (large clusters)
What happens if a protein misfolds?
Misfolded proteins can aggregate, forming problematic structures such as plaques or aggregates that disrupt cellular function.
Neurodegenerative diseases like Alzheimer's are often associated with the accumulation of these misfolded proteins, leading to cell death and loss of function.
Sometimes, proteins can be refolded back from the incorrect conformation
How do you denature a protein?
Chemical denaturation using urea
Disrupts hydrophobic interactions, diminishing the hydrophobic effect
Thermal denaturation; heating the proteins. Often irreversible if aggregates form
Like cooking an egg; proteins unfold, aggregates form
Disulfide bonds can also cause an issue though because when unfolded due to heat, two cysteine residues form disulfide bonds, locking in the proteins shape (this what causes eggs to solidify)
What are Protein Families?
Groups of proteins that share similar structures and functions, often arising from a common evolutionary ancestor. These families may perform related biological roles or exhibit similar sequences.
Some residues are conserved within a family because they are critical for function or structure. If highly conserved residue is changed, can disrupt function
Explain Sized based Separation
Separates proteins by size
Larger proteins elute earlier because they can’t enter the pores of the beads
Smaller proteins enter the pores and take longer to pass through, eluting later
Explain Ion-Exchange Chromatography
Anion Exchange:
beads carry positive charge and negatively charged proteins bind to it, everything else passes through. These now bounded proteins can be eluted by increasing the salt concentration
Cation Exchange:
Same thing, just beads carry a negative charge
Explain Affinity-based chromatography
Columns are prepared with a ligand or binding partner for the target protein to bind to. Everything else will elute through the column.
Target protein can be eluted by disrupting the binding