lecture 5 protein structure
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
primary structure
the sequence of amino acid monomers in the polypeptide.
secondary structure
the local folding of the polypeptide into repeated patterns. There are two kinds of secondary structure: • α (alpha) helix and β (beta) pleated sheet.
alpha helix
right handed coil
β (beta) pleated sheet
two or more sequences are extended and aligned
hydrogen bonds
secondary structure held together by _______ between the amide H and the carbonyl O in the polypeptide backbone (the R groups are not involved).
peptide bonds (covalent bonds)
primary structure is held together by _____
Tertiary structure
the final folded structure of the polypeptide into a functional protein
a variety of bonds and forces
Tertiary structure held together by _______ between the R groups, but may include interactions between the R groups and the polypeptide backbone.
covalent bonds (disulfide bonds) • ionic bonds • hydrogen bonds • van der Waals interactions • hydrophobic interactions
a variety of bonds and forces for tertiary structure
quarternary structure
results from the ways in which two or more polypeptide chains (subunits) bind together and interact
same as tertiary
Quarternary structure help together by_____
denaturing
heat or chemicals disrupt weak interactions in a protein, destroying secondary and tertiary structure. may be able to return to normal when cooled or the chemicals are removed.
Protein functions
Determined by binding characteristics: Enzymes—catalytic molecules • Structural and motor proteins • Signal and regulatory proteins • Receptor proteins • Transport proteins • Defensive proteins • Storage proteins
ligand
molecule or ion that binds to another molecule. binding is specific and can involve multiple weak bonds, making a relatively strong interaction
binding affinity
strength of the interaction
shape of the portein
Ligand binding changes the ____. This causes the protein to become active (or inactive) depending on the specific protein).
R group modification
changes the protein’s shape or create or hide a ligand binding site. This causes the protein to become active (or inactive) depending on the specific protein)
Proteolysis
can also unmask a ligand binding site or create a binding site for other proteins. • This causes the protein to become active (or inactive) depending on the specific protein).
by proteolysis
some proteins are activated ____
addition of cofactor
Some proteins require a non-protein molecule or ion for function. These nonprotein molecules or inorganic ions (often a metal ion), are called _______ . Cofactors includes coenzymes and ATP
lower the activation energy
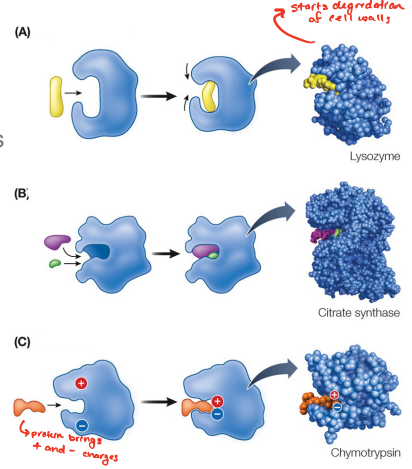
Enzymes _______ in almost all chemical reactions in cells. Enzymes do this through binding to their substrates • The formation of bonds releases free energy. • This free energy is used to change the shape of the enzyme and/or break bonds in the substrate.
subtrates ; active sites
Reactants are _____ : they bind to specific sites on the enzyme—the ______
change shape
Many enzymes _____ when the substrate binds
hydrogen bonding, ionic bonds, van der Waals interactions, or temporary covalent bonding
The enzyme–substrate complex (ES) is held together by
• inducing strain • substrate orientation • or by adding chemical groups
There are several mechanisms by which enzymes lower the activation energy. These include:
catabolic pathways
break down molecules into smaller molecules and release energy
anabolic pathways
synthesize complex molecules from simpler ones.
inhibitors
are molecules that bind to the active site, preventing the substrate from entering
rare ; reversible
Irreversible inhibition is ______, mostly occurs with certain drugs. Competitive inhibition is ______, inhibitor is similar to the substrate, but no reaction occurs.
allosteric regulation
nonsubstrate molecule binds to a site other than the active site
phosphorylation
a phosphate group is added by a protein kinase; in a hydrophobic region of the enzyme, the altered amino acid may induce a change to interact with hydrophilic regions.
protein phosphatase
remove phosphate groups from proteins.
glycosidic linkage
which interaction does NOT determine tertiary structure in proteins
secondary and tertiary
which levels of protein structure would be destroyed by the addition of denaturing reagent?