D515 Molecular Cell Bio Lecture 4: Cellular Signaling Pathways
1/103
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
104 Terms
Pro-inflammatory Proteins and Lipids
-promote inflammation, which is a protective response by the body to injury or infection
-ex: cytokines like TNF-α, IL-1, and IL-6.
Anti-inflammatory Proteins and Lipids
-molecules work to reduce inflammation and restore normal tissue function after the initial immune response.
-include cytokines TGF-β
hormones
-signaling molecules produced by glands in the endocrine system and released into the bloodstream to regulate various physiological processes.
-can act on distant target cells to regulate functions like metabolism, growth, and immune response.
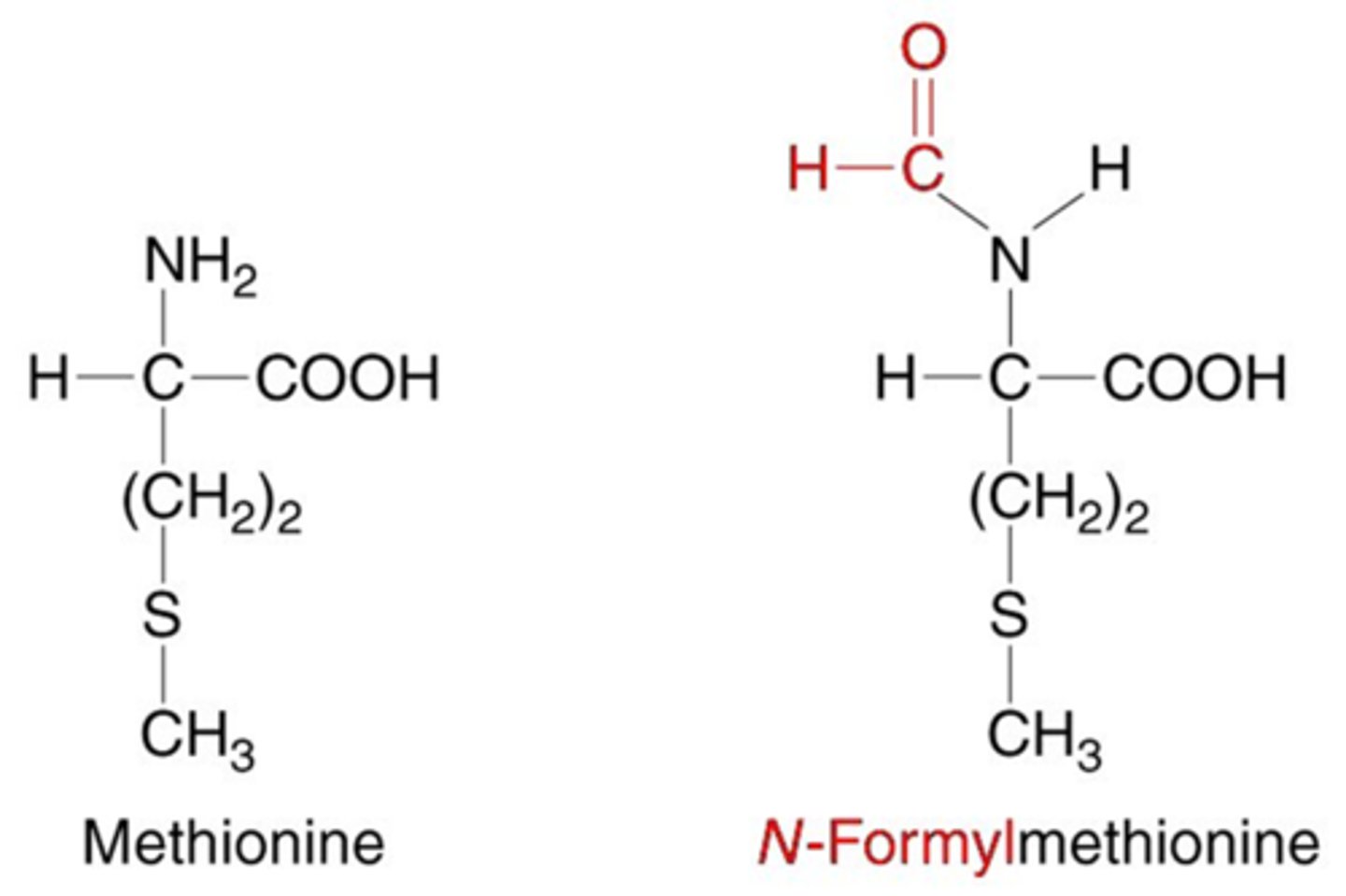
N-formylmethionine
-modified form of the amino acid methionine
-the first amino acid incorporated during the synthesis of proteins in prokaryotes (bacteria)
-recognized by the immune system as signals of bacterial infection and can trigger an immune response
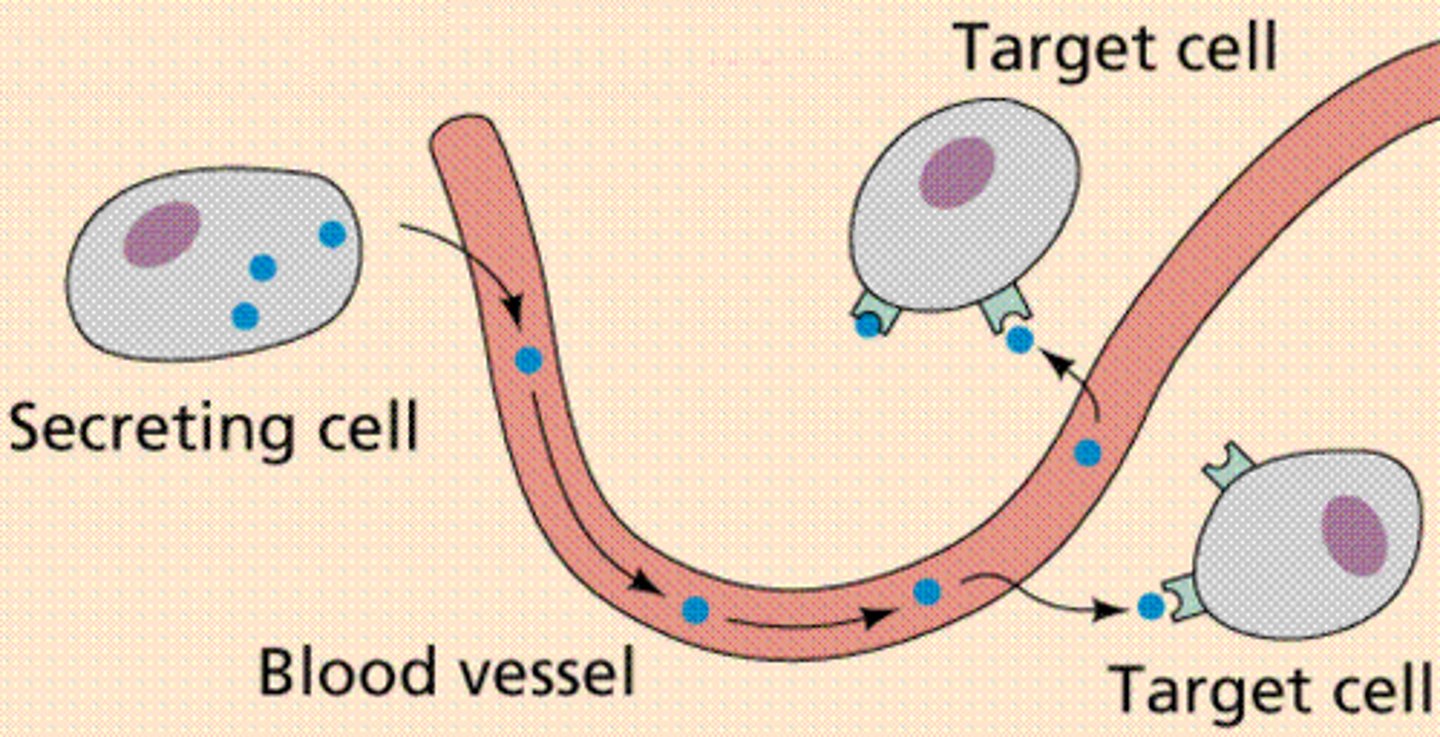
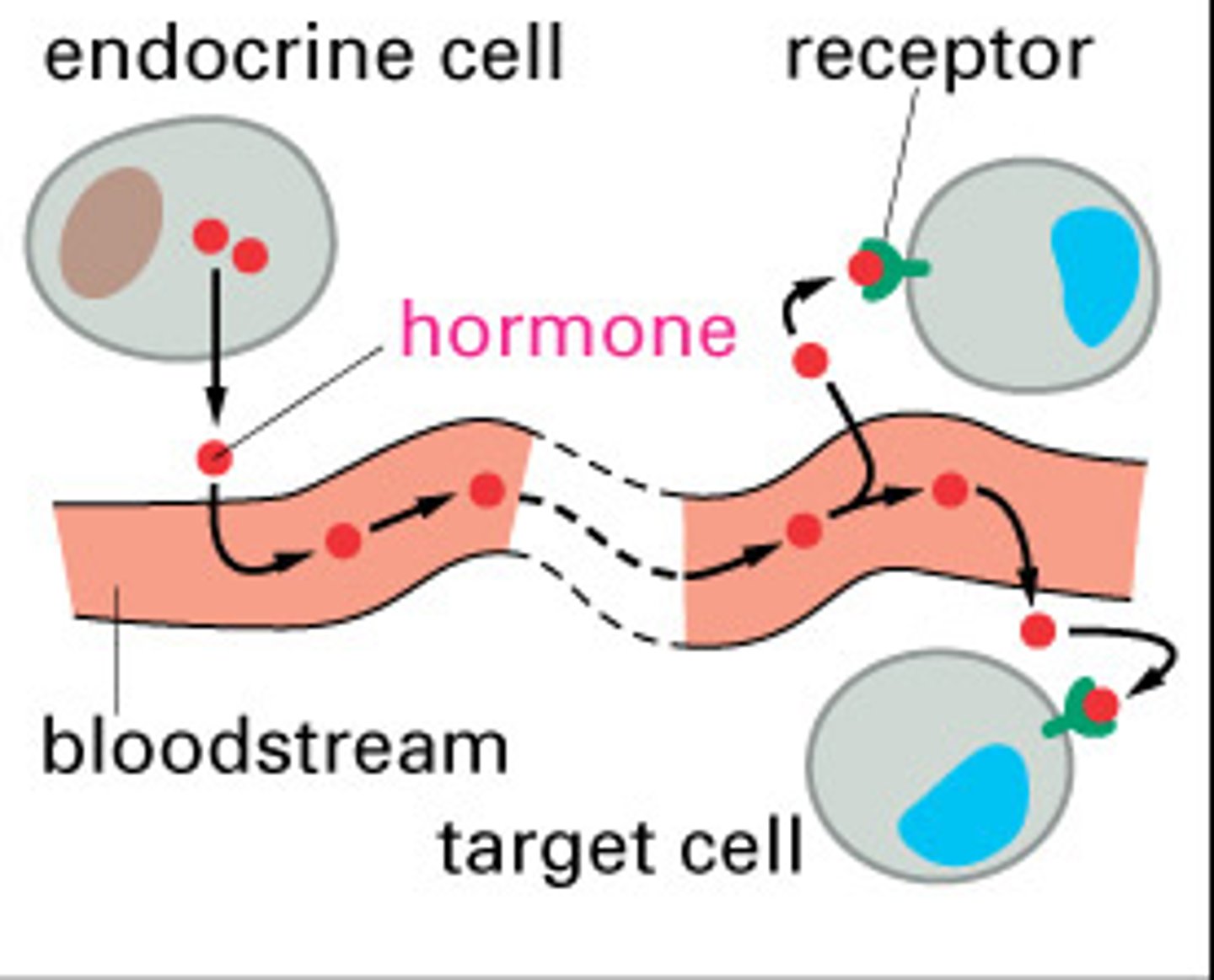
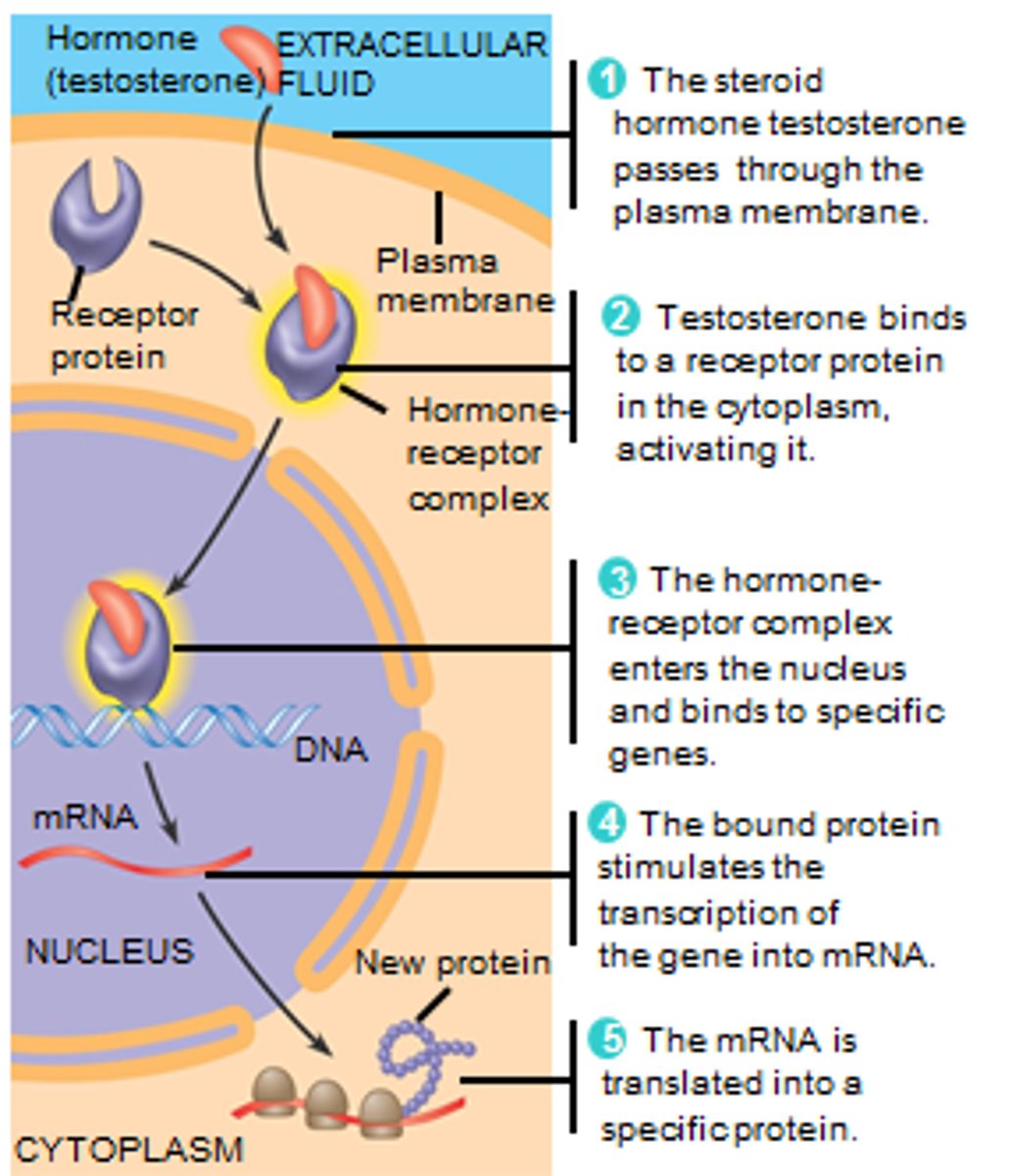
endocrine signaling
-signaling molecules (hormones) are released by endocrine cells into the bloodstream
-hormones travel long distances to reach distant target cells
-ex: Insulin is released by the pancreas and regulates blood glucose levels throughout the body
-Long-range signaling and slow response compared to other signaling types
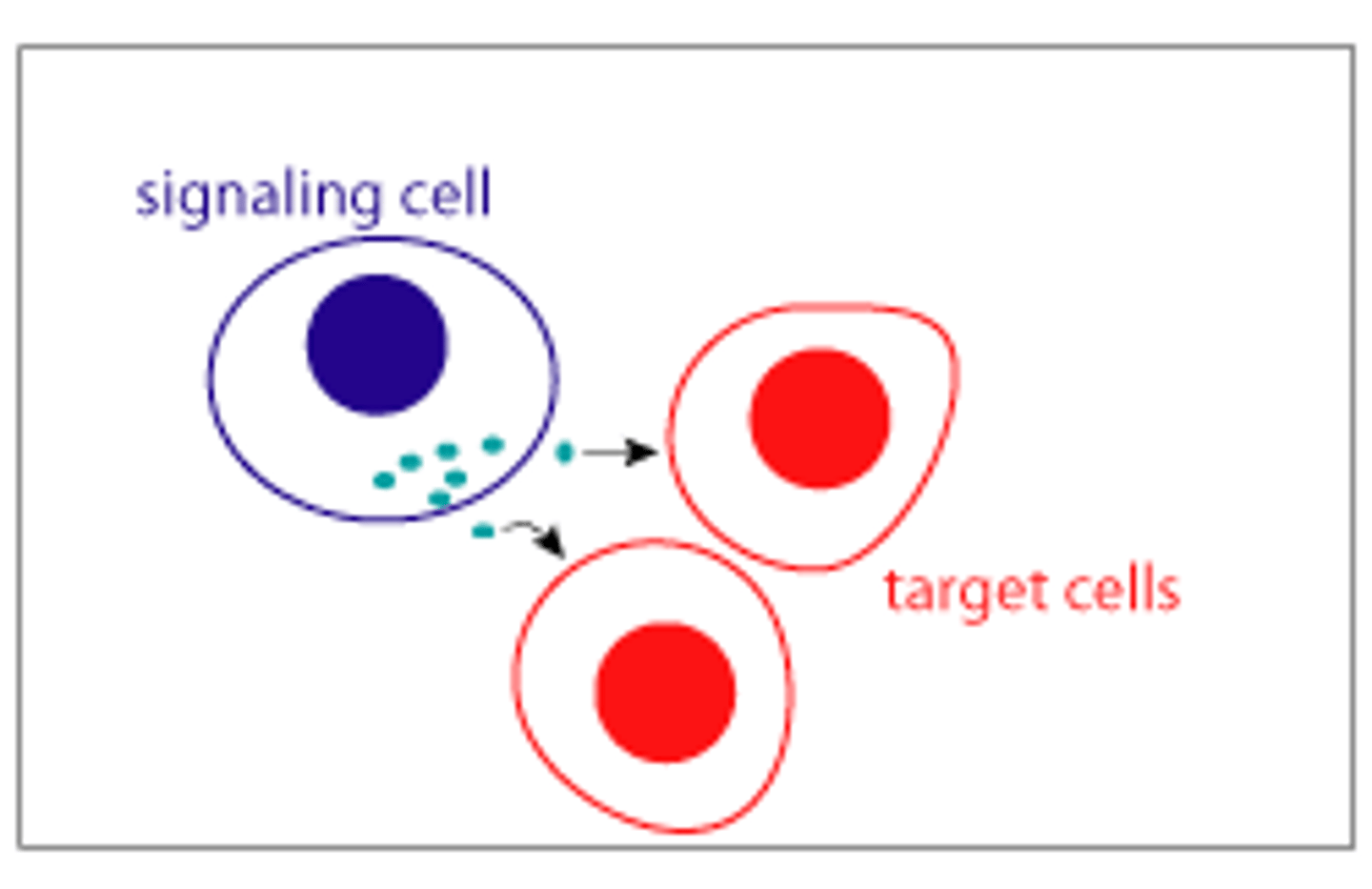
paracrine signaling
-signaling molecules are released by a cell & act on neighboring cells in the local environment
-Ex: Neurotransmitters are released by neurons at synapses and act on adjacent neurons or muscle cells.
-Short-range signaling & quick response due to the close proximity of target cells
-Important in processes like tissue repair and immune responses
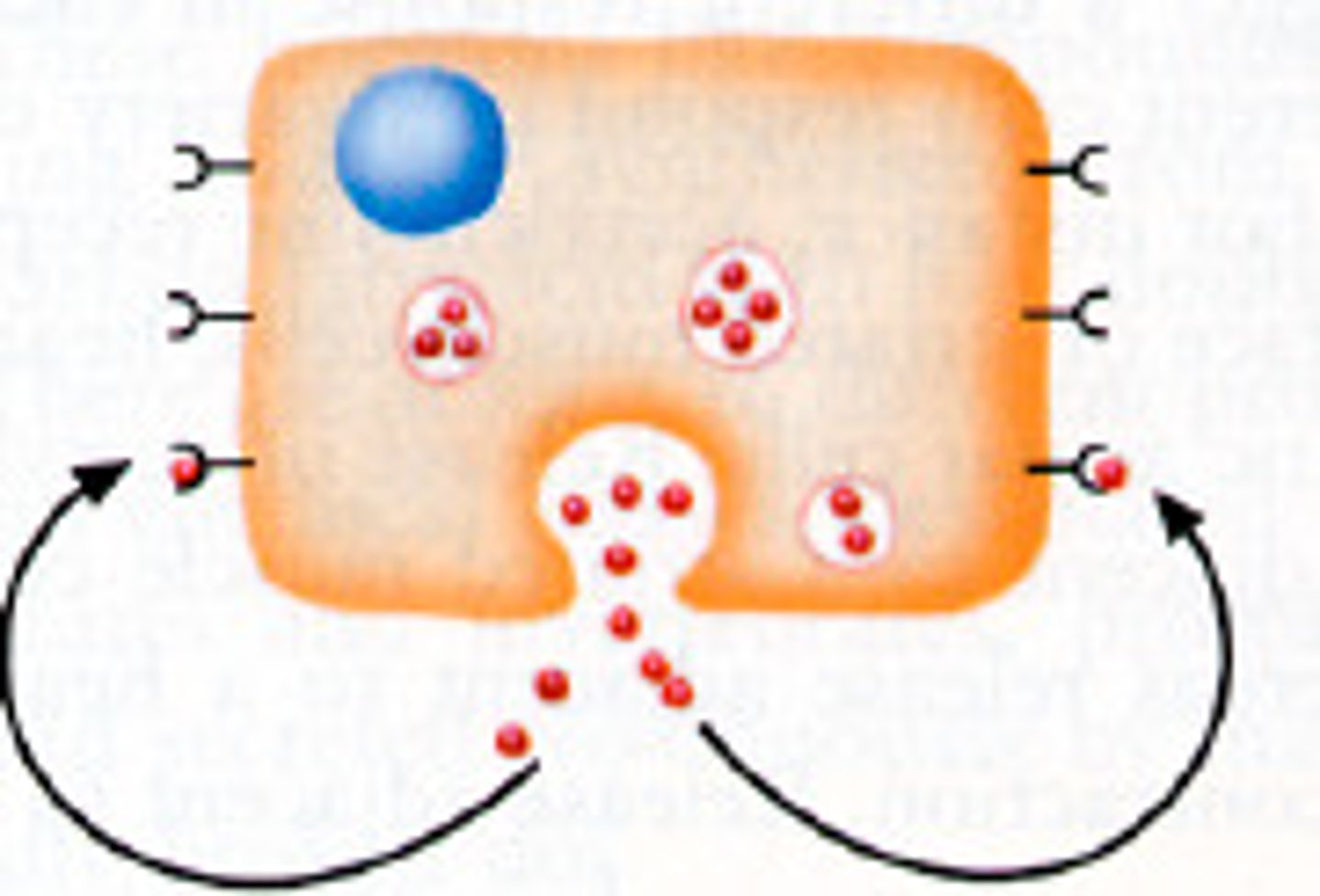
autocrine signaling
-a cell releases signaling molecules that bind to receptors on its own surface, leading to changes within the same cell
-Ex: some immune cells release cytokines that act on themselves to promote their own activation or proliferation.
-signaling cell is also the target cell, often involved in self-regulation processes
-can amplify responses, especially in the immune system
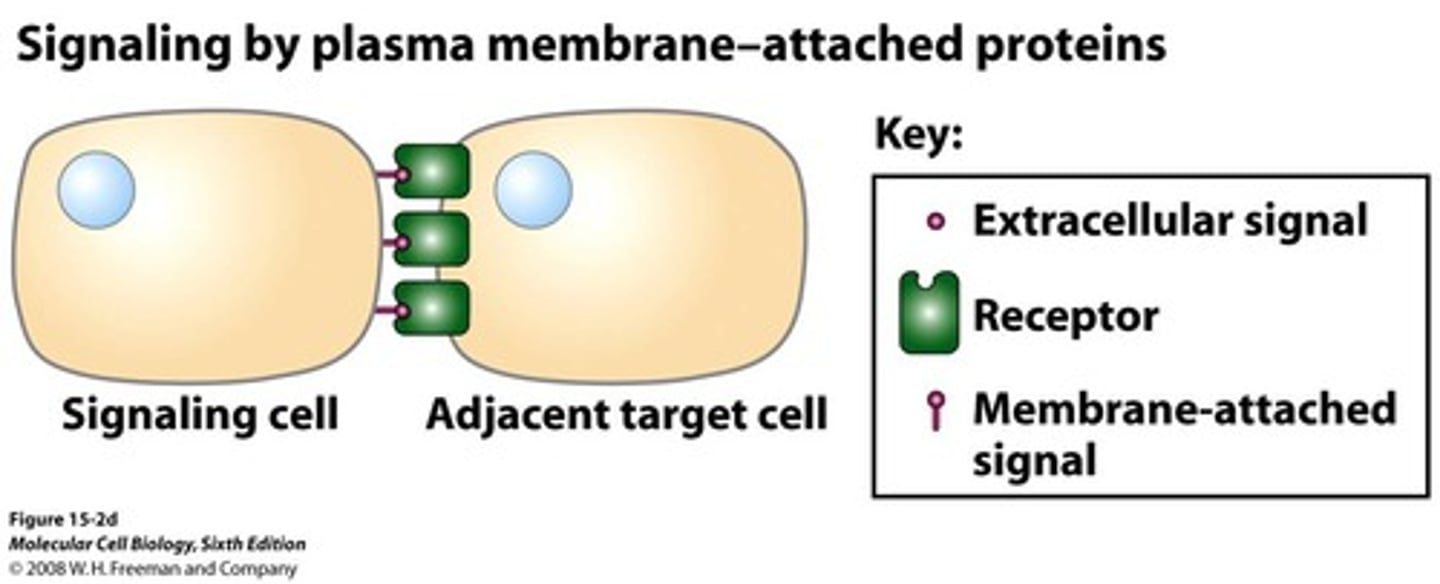
signaling by plasma-membrane-attached protiens
-signaling molecules are not released into the extracellular space. Instead, they remain attached to the plasma membrane of the signaling cell and interact directly with receptor proteins on adjacent cells
-ex: Immune cell interactions include T cells and antigen-presenting cells communicate through direct contact via membrane-bound proteins.
-Requires direct cell-to-cell contact
Direct Cell-Cell contact via Plasma Membrane Receptors
-Plasma membrane receptors on one cell bind to specific ligands (usually proteins) on the surface of an adjacent cell. This binding triggers intracellular signaling pathways within the interacting cells
-important in embryonic development & the immune system (T cells)
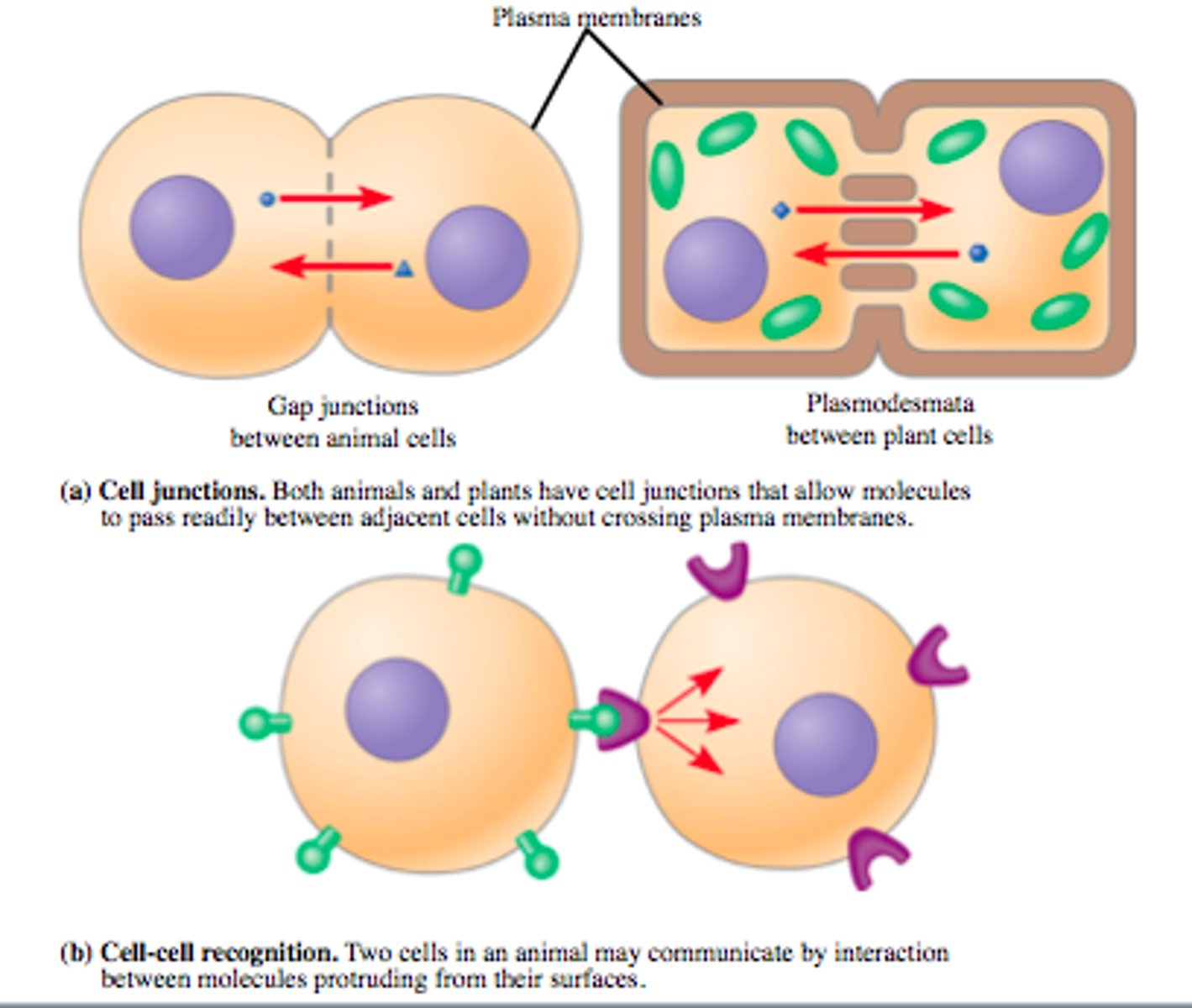
Direct Cell contact via Cell Junctions
-Gap Junctions: specialized connections between adjacent cells that allow for the direct exchange of ions, nutrients, and other small molecules.
-Communication involves direct touching of cells or indirect communication via secreted chemicals
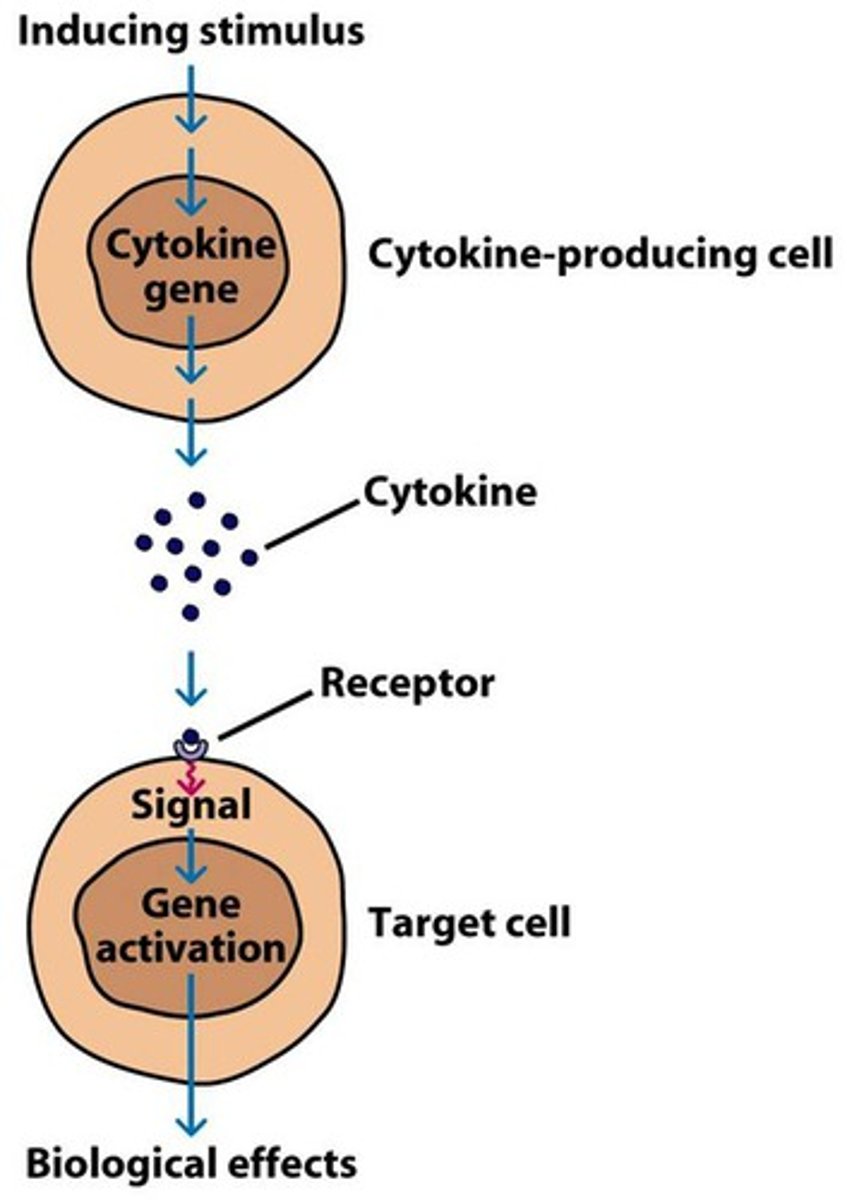
Steps involved in Indirect Communication via Chemical Messengers
1. Synthesis and Release of Signaling Molecules
2. Transport of the Signaling Molecule to the Target Cell
3. Binding of the Signaling Molecule to Receptors on the Target Cell
4. Signal Transduction Pathways
5. Cellular Response
6. Removal of the Signal
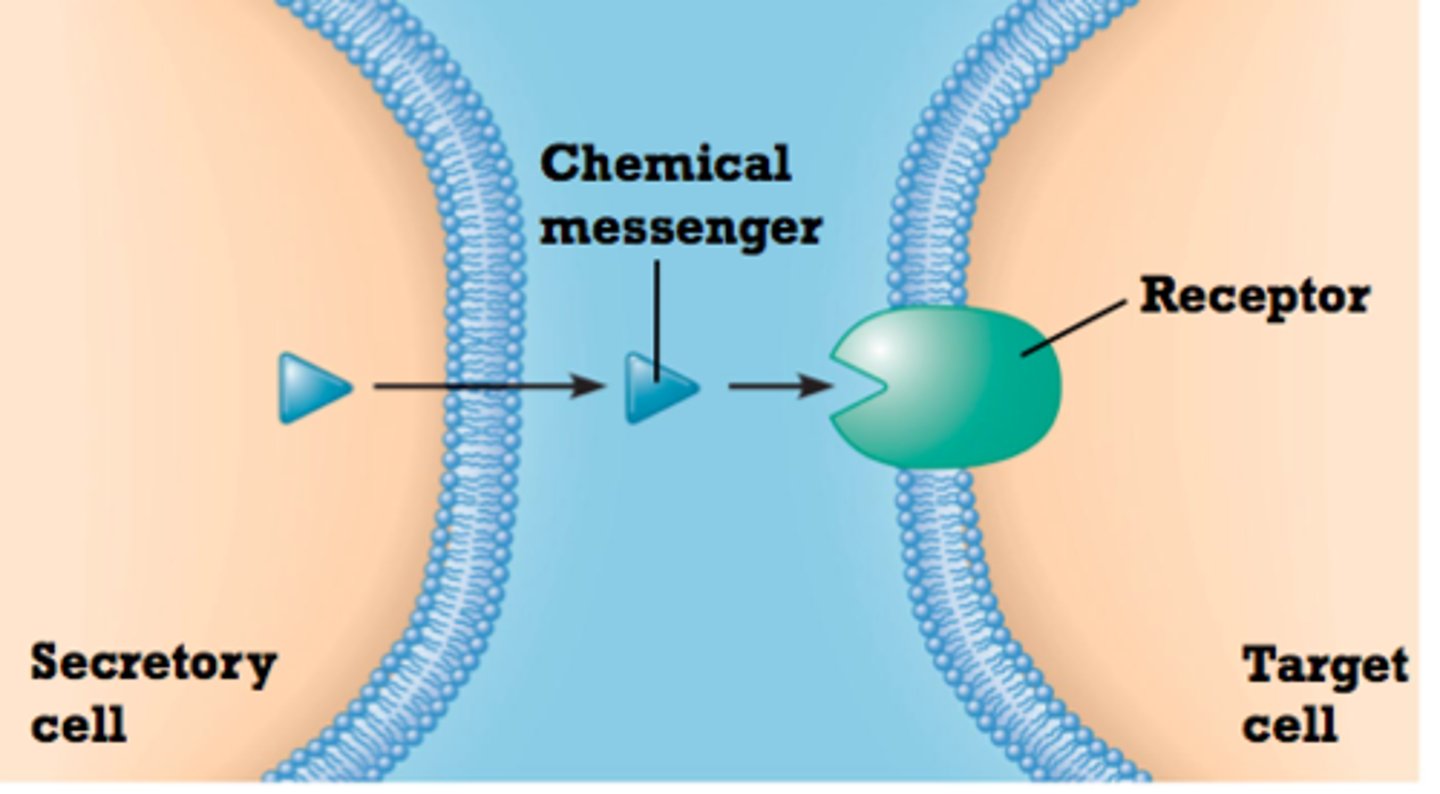
Synthesis and Release of Signaling Molecules (indirect communication)
-A signaling cell synthesizes and releases a signaling molecule (e.g., a hormone)
-This occurs through the secretory pathway, where the molecule is processed and transported to the cell membrane for release.
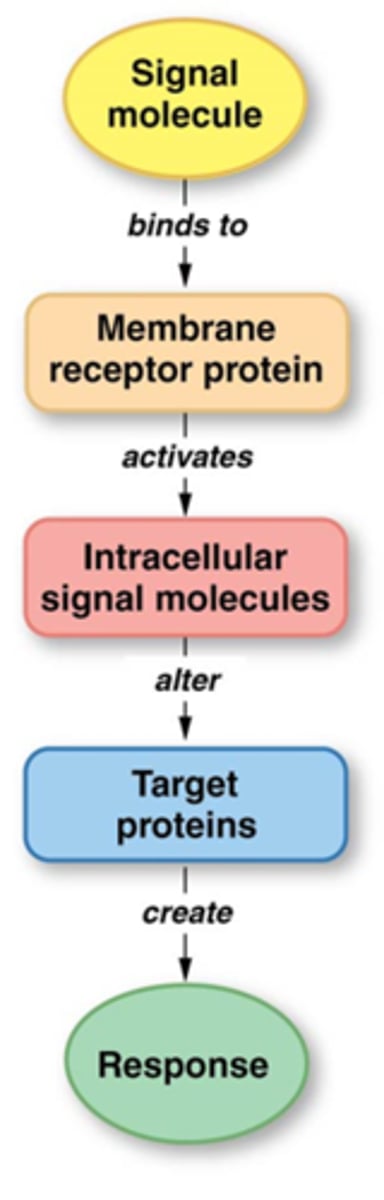
Effects of Binding of the Signaling Molecule to Receptors on the Target Cell
- This binding leads to receptor activation by causing:
- A change in the receptor's 3-D conformation
- Receptors aggregate together
- Receptor movement through/within the cell
Signal Transduction
-signal is relayed from one part of the cell (e.g., the plasma membrane) to another part (e.g., the nucleus).
-process by which a cell responds to external signals, leading to various cellular responses
-can involve changes in existing proteins or alterations in gene transcription.
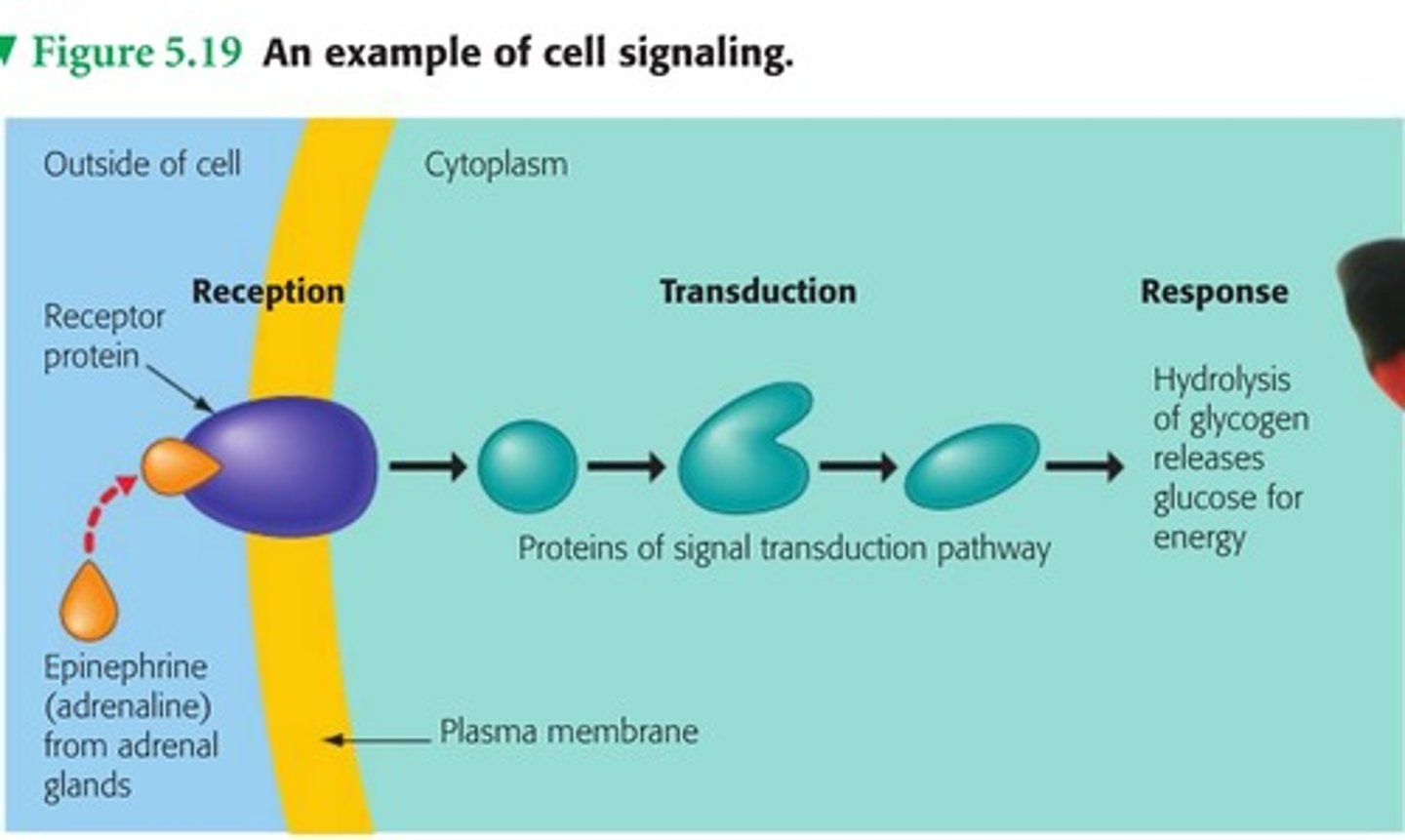
Cellular Response
-end result of signal transduction
-can be an immediate change in the activity of existing proteins or a longer-term change in gene transcription
consequence of not removing the signal once the desired response is achieved
-If the signal (e.g., for cell division) is not removed, it can lead to uncontrolled cell growth.
-Persistent signaling is characteristic of many cancers due to abnormally activated signaling pathways.
Common Themes in Cell Signaling Pathways
1. One receptor can activate multiple pathways, leading to different responses.
2. One pathway can induce multiple responses (e.g., activating many genes).
3. Limited number of core pathways (5-10) integrate all environmental signals.
4. Different pathways can lead to the same cellular response.
Pathways may modify pre-existing proteins or regulate transcription.
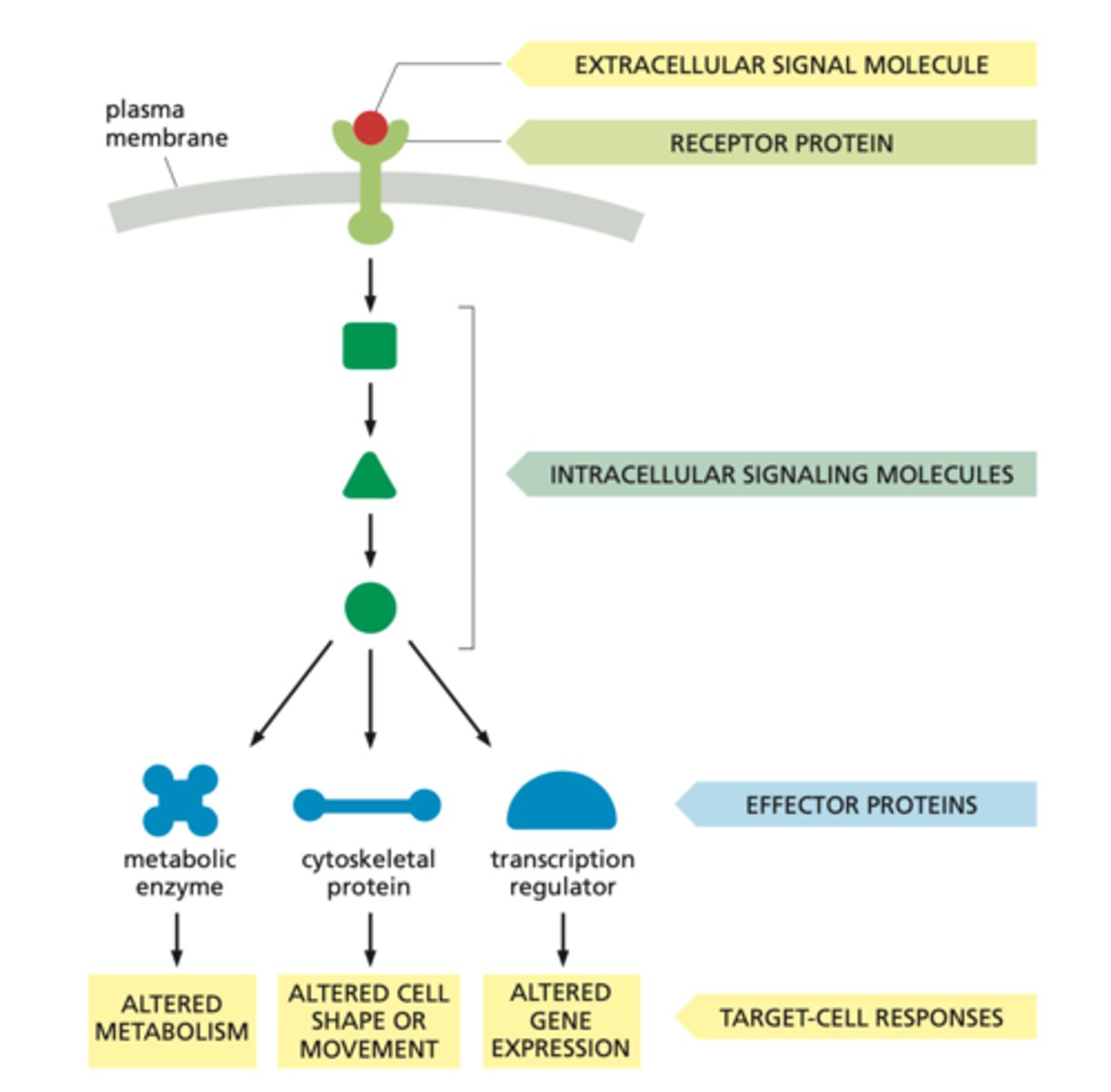
Multiple Pathways Activated by One Receptor (common theme 1)
-Some receptors can activate more than one signaling pathway.
-This can lead to different cellular responses depending on which pathway is activated.
-ex: A single receptor might trigger pathways that lead to both cell growth and immune response, depending on the context.
Multiple Responses from One Pathway (common theme 2)
-Concept: A single signaling pathway can induce multiple cellular responses.
-Example: If a pathway activates a transcription factor, that transcription factor may turn on 30 different genes, leading to various outcomes like cell growth, differentiation, or apoptosis.
Limited Number of Core Signaling Pathways (common theme 3)
-It is believed that there are only 5-10 core signaling pathways.
-All environmental signals are thought to feed into these limited pathways, activating them in different combinations to produce a wide variety of cellular responses.
Different Pathways Converging on the Same Response (common theme 4)
-Different signaling pathways can lead to the same cellular response.
-ex: A cytokine receptor pathway might activate cell division in T cells and the NFκB pathway might induce the same cell division response in fibroblast cells.
-Implication: Different stimuli can lead to the same biological outcome through different mechanisms.
What are the two possible outcomes of signaling pathways? (common theme 5)
-Modification of pre-existing proteins (e.g., phosphorylation).
-Regulation of transcription (production of new proteins).
What is the specificity of receptor-ligand binding?
-Most receptors only bind to a single type of ligand.
-Binding is highly specific, similar to the enzyme-substrate "lock-and-key" model
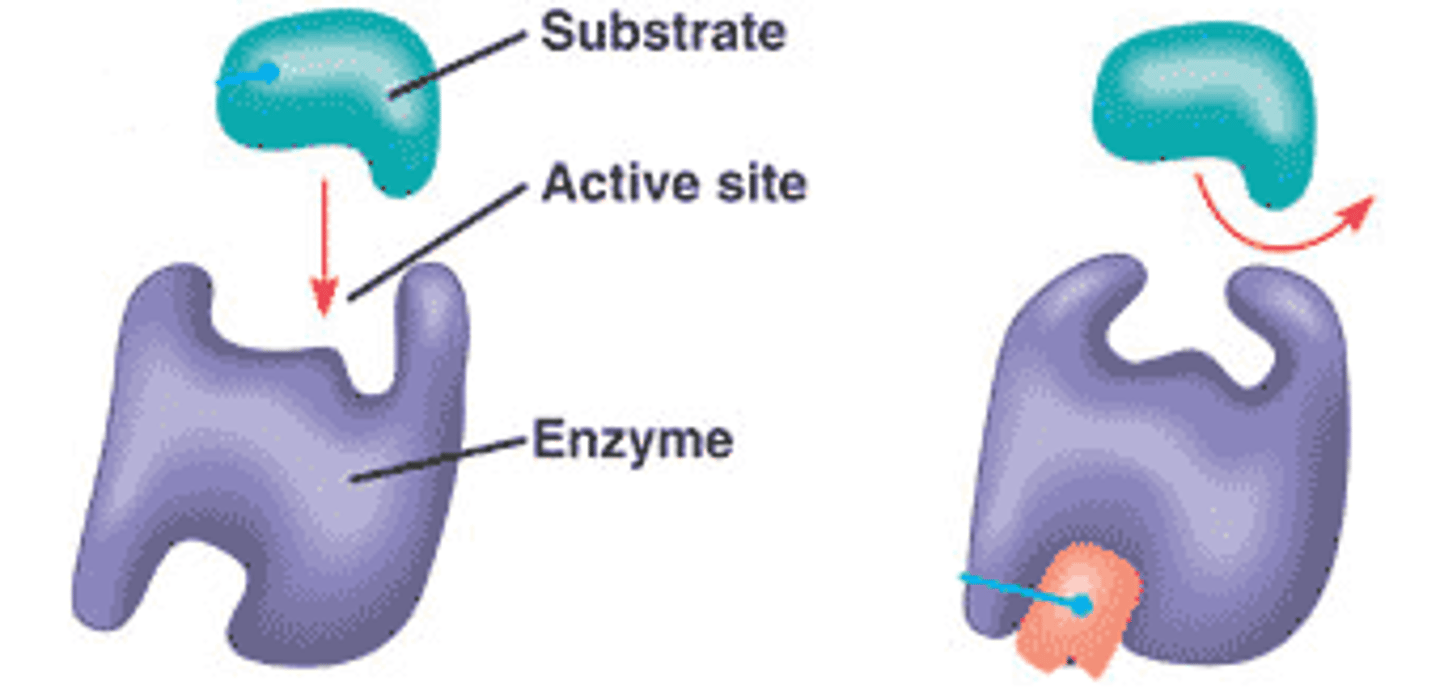
Do all receptors activate the same signaling pathways?
-No, each receptor type activates specific pathways.
-Example: The IL-4 receptor activates the JAK/STAT pathway but not the MAP kinase pathway.
How do ligands bind to their receptors?
-via hydrogen bonding, ionic bonds, van der Waals forces, and hydrophobic interactions
-create a "perfect fit" between the ligand and receptor.
Is 100% receptor binding necessary for a cellular response?
No, more than one ligand binding to receptors is needed, but not all receptors must be bound to trigger a response
How does receptor density affect cellular sensitivity to ligands?
-Fewer receptors require more ligand to elicit a response
-Cells can reduce sensitivity by internalizing receptors
-Example: HER2 receptor density affects response to EGF in breast cancer.
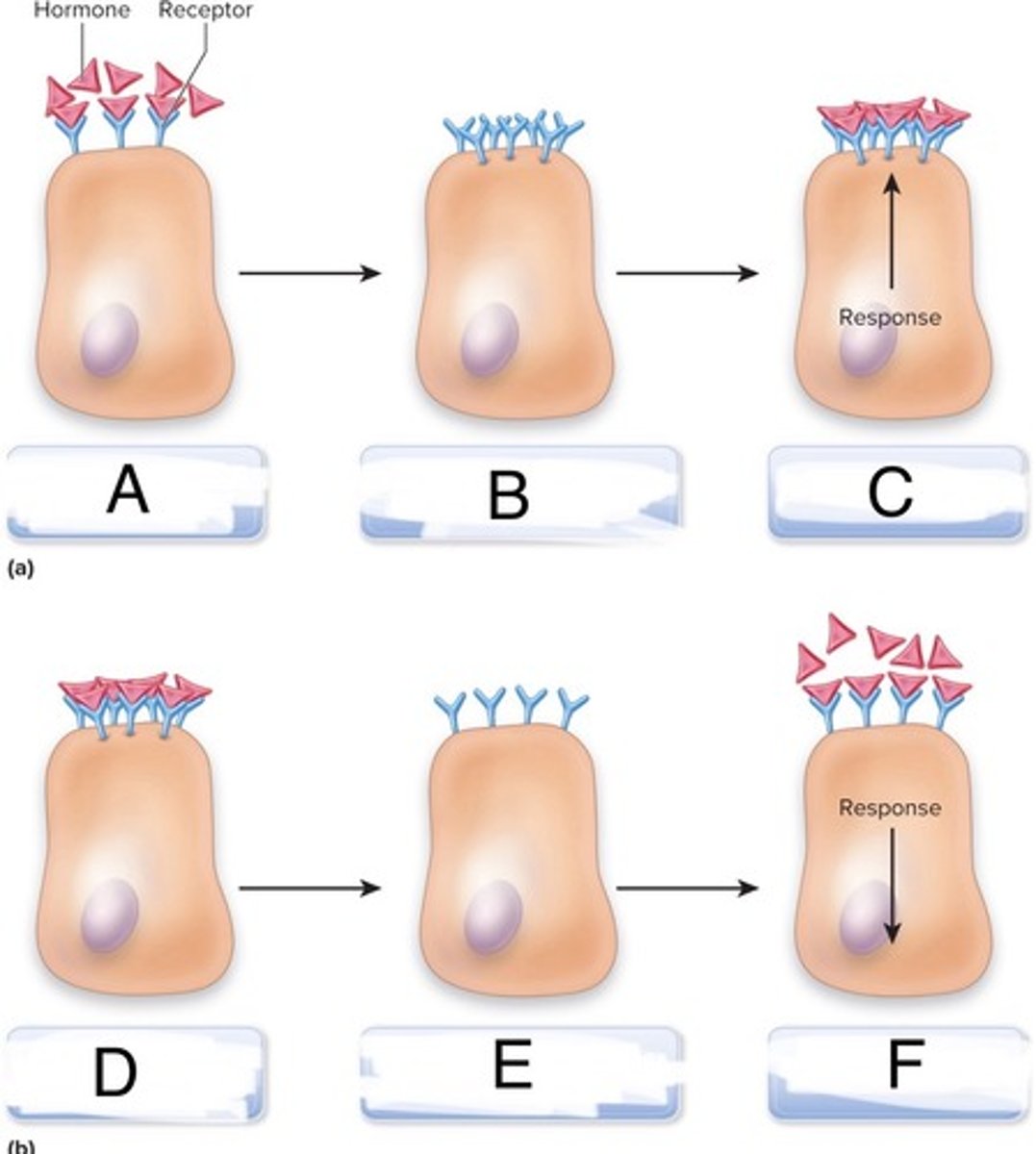
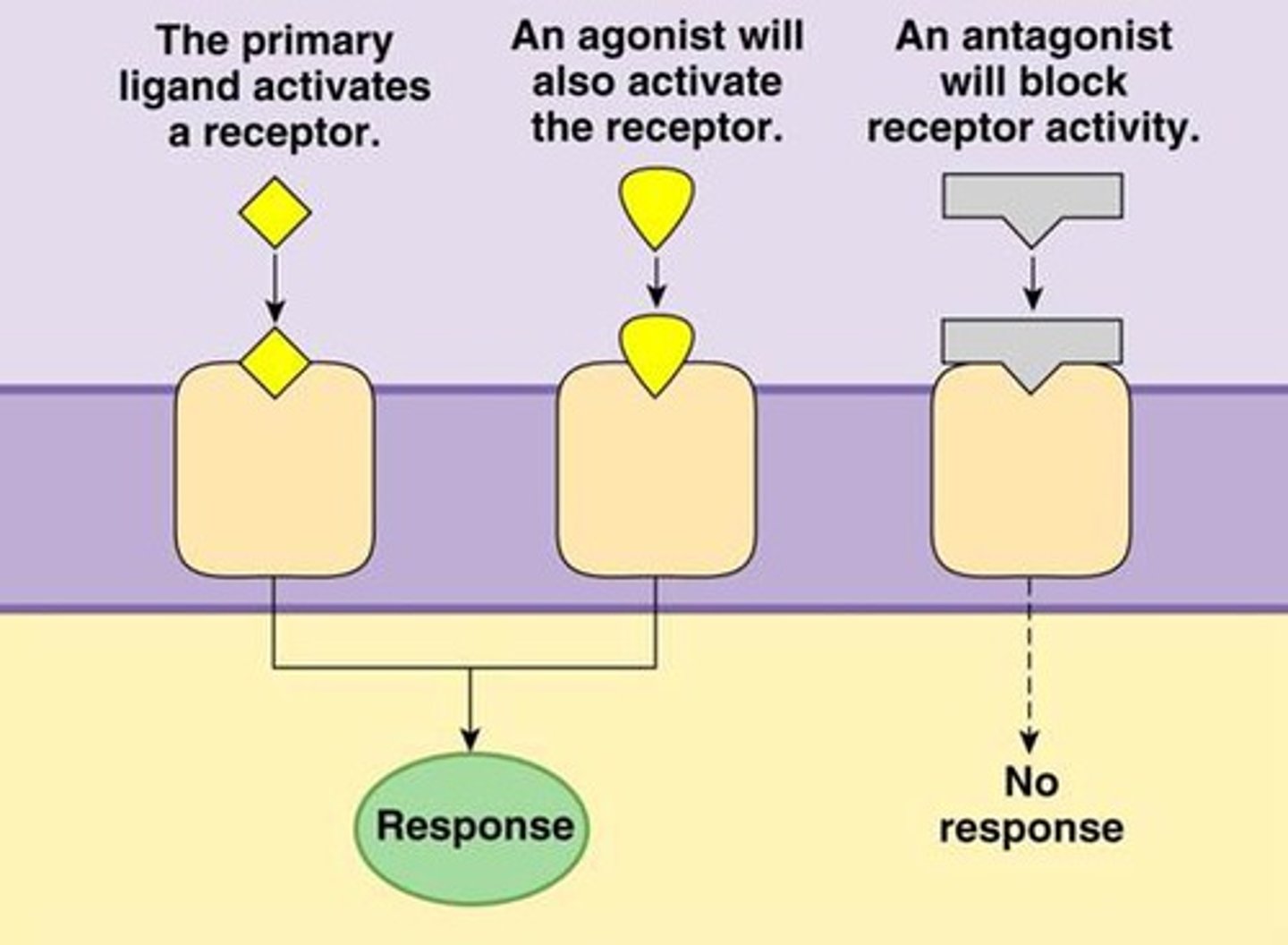
agonist
-Molecules that resemble the ligand and induce the cellular response.
-Some agonists can have a higher affinity for the receptor than the natural ligand, leading to a stronger cellular response.
-ex: Isoproterenol (chemically synthesized) is an agonist used in the lungs that can induce a more potent response than epinephrine (natural ligand)
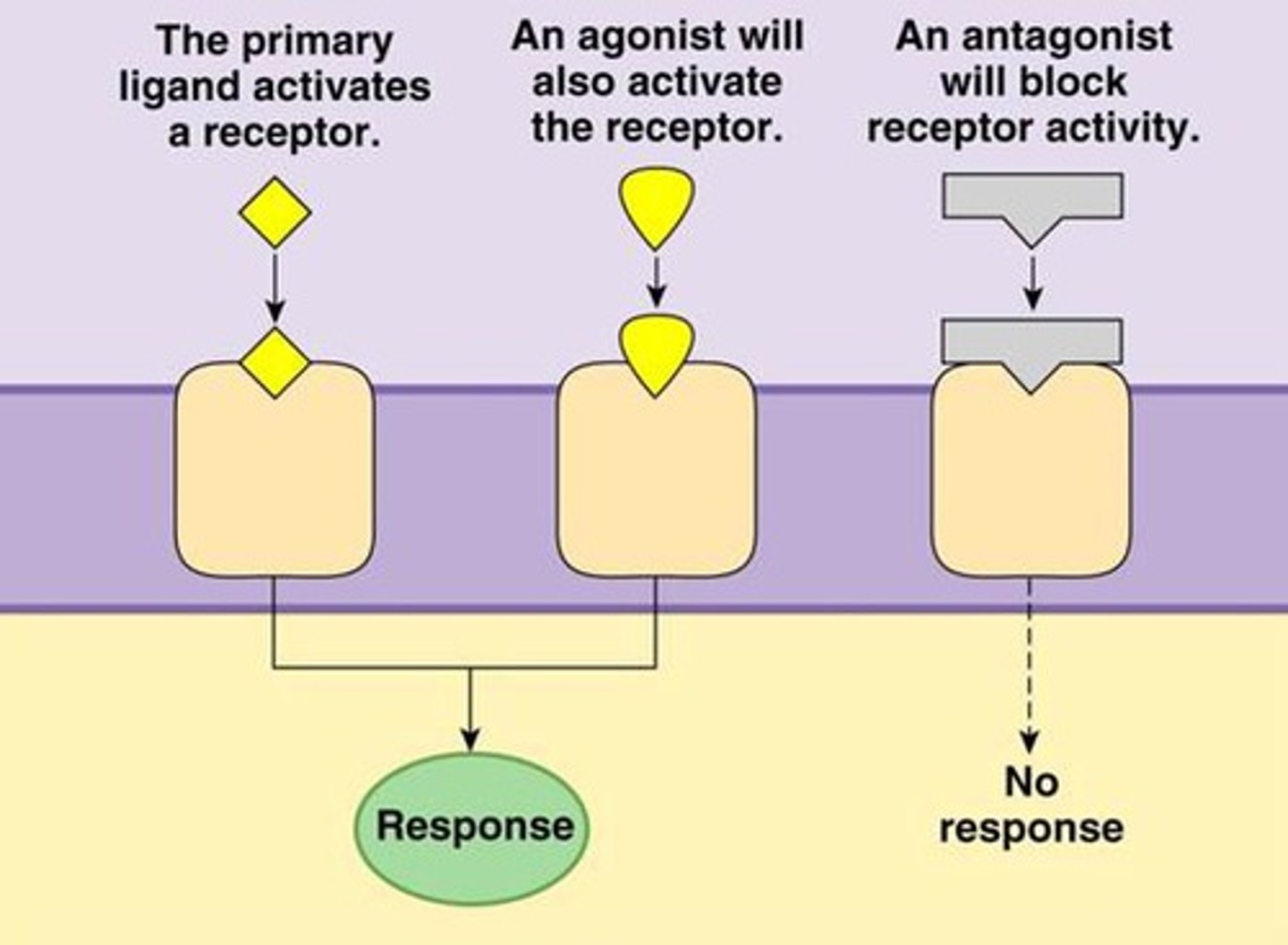
antagonist
-bind to the receptor but do not induce a response, blocking the receptor.
-act like competitive inhibitors.
-ex: Alprenolol in the heart blocks the effects of epinephrine.
Fingolimod
-antagonist that inhibits sphingosine-1-phosphate (S1P) receptors
-anti-inflammatory effects, specifically by reducing the activity of certain immune cells that contribute to inflammation.
-synthetic derivative of a compound naturally found in fungi
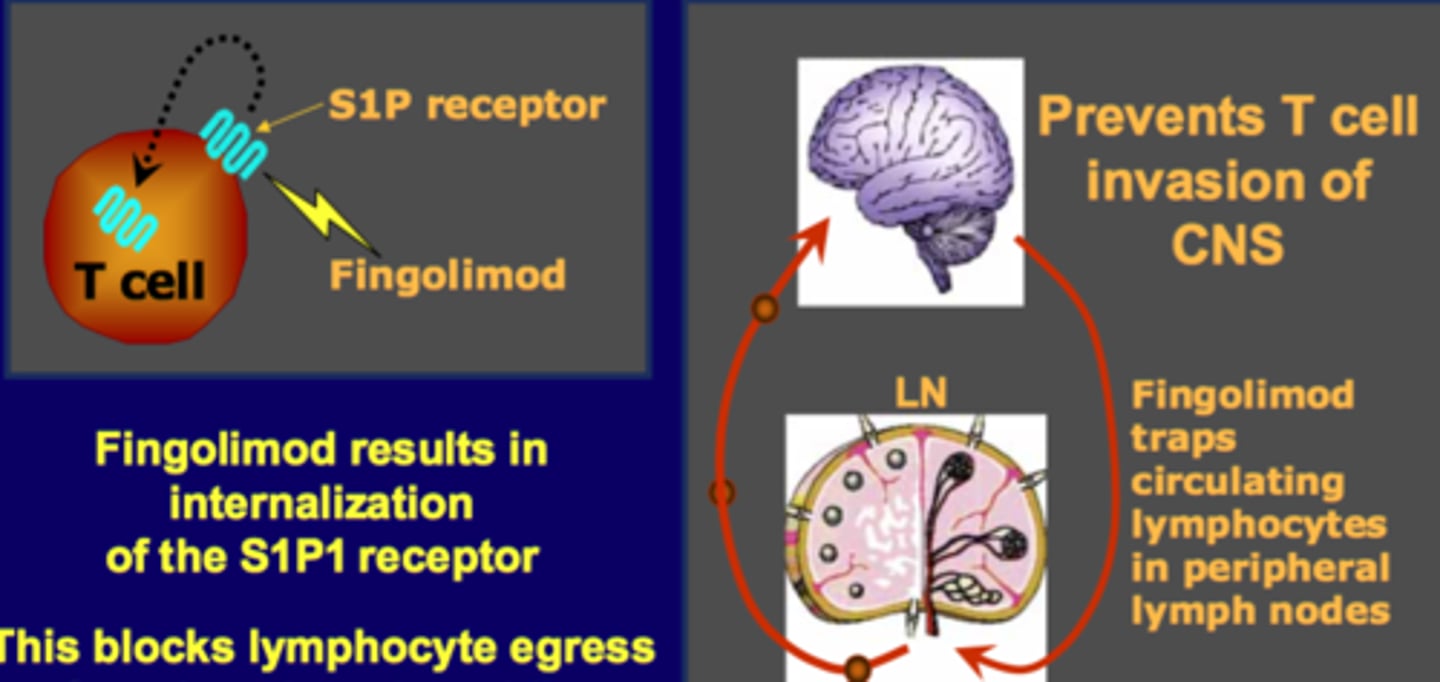
Sphingosine-1-Phosphate (S1P)
-signaling lipid that binds to S1P receptors on cells, playing a role in immune cell trafficking and other cellular processes.
-By inhibiting S1P receptors, Fingolimod reduces the movement of immune cells, thereby reducing inflammation and autoimmunity
What initiates intracellular signaling pathways?
They are initiated when a ligand binds to a specific receptor on the cell membrane.
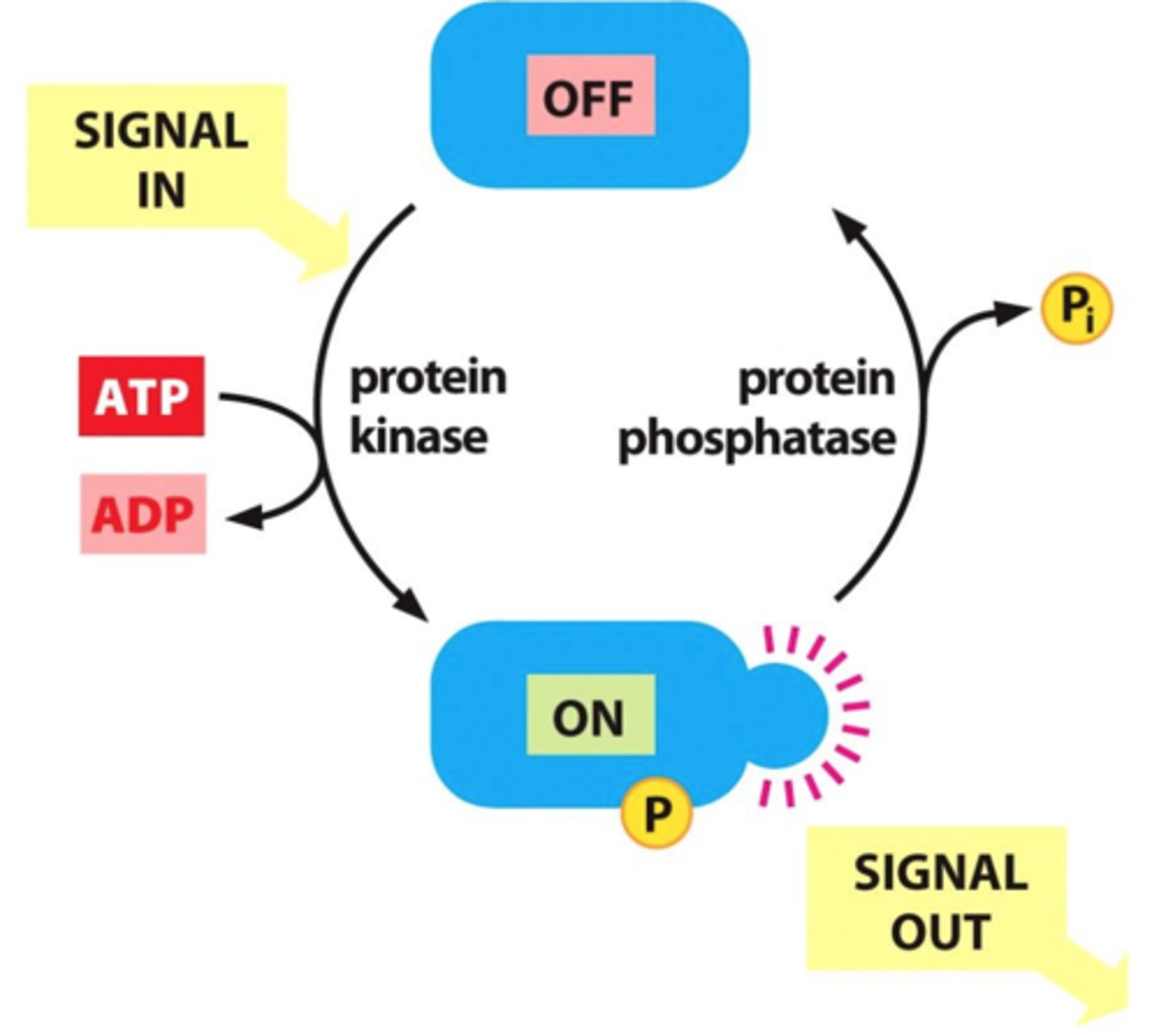
What role do kinases play in intracellular signaling?
add phosphate groups to target proteins (phosphorylation), often leading to their activation and triggering a signaling cascade
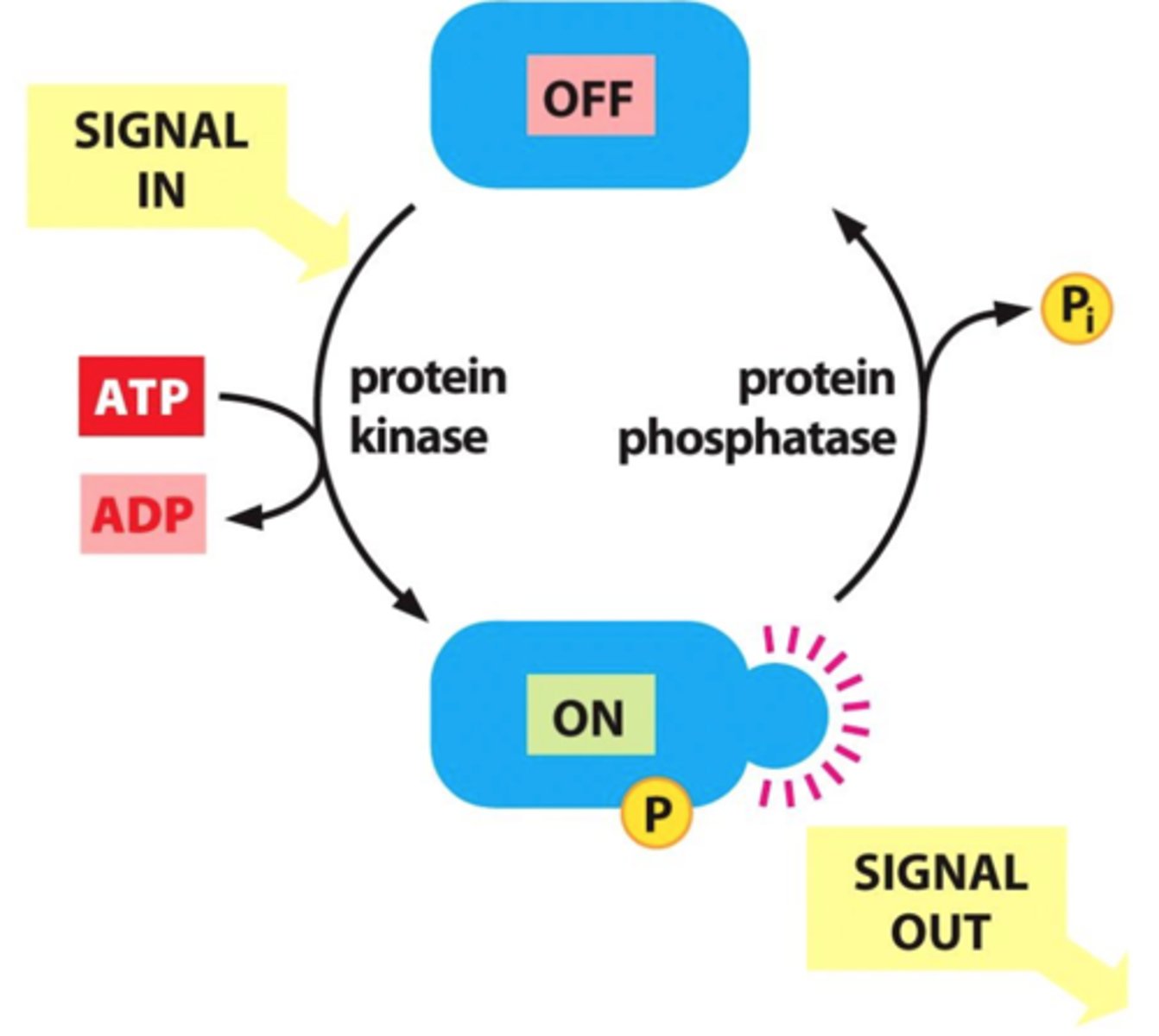
How do phosphatases regulate intracellular signaling?
remove phosphate groups from proteins (dephosphorylation), which can turn off signals and regulate the duration and intensity of the response.
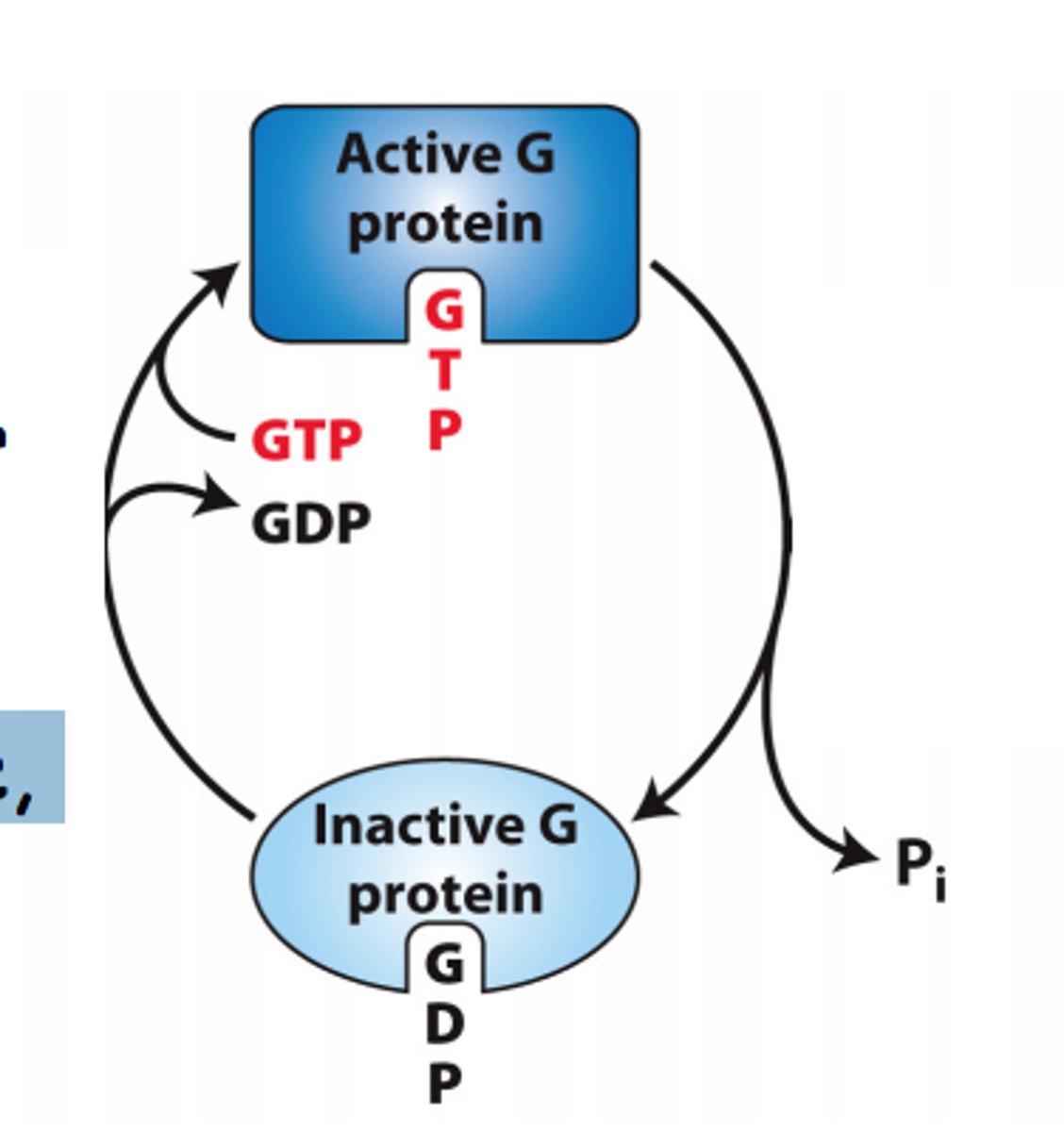
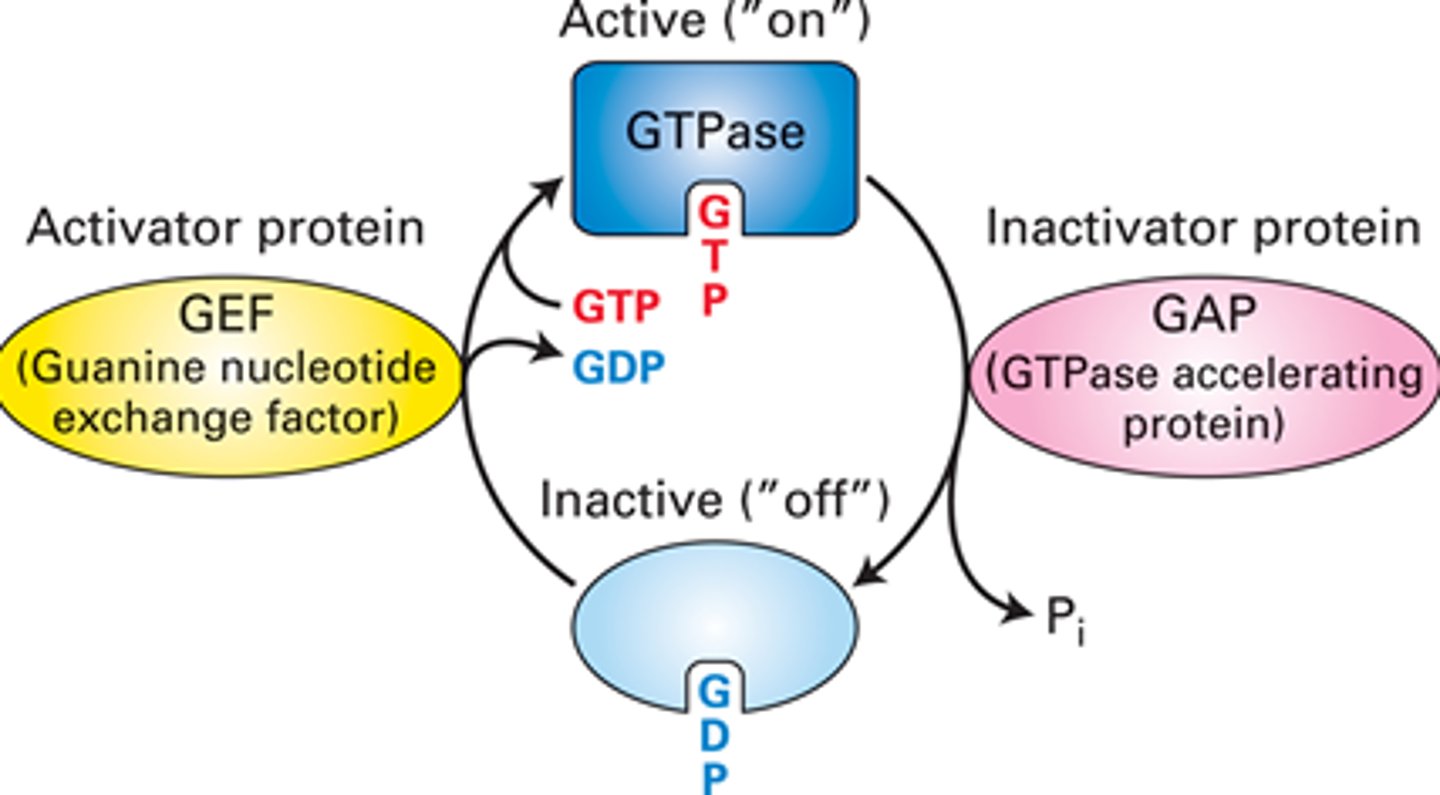
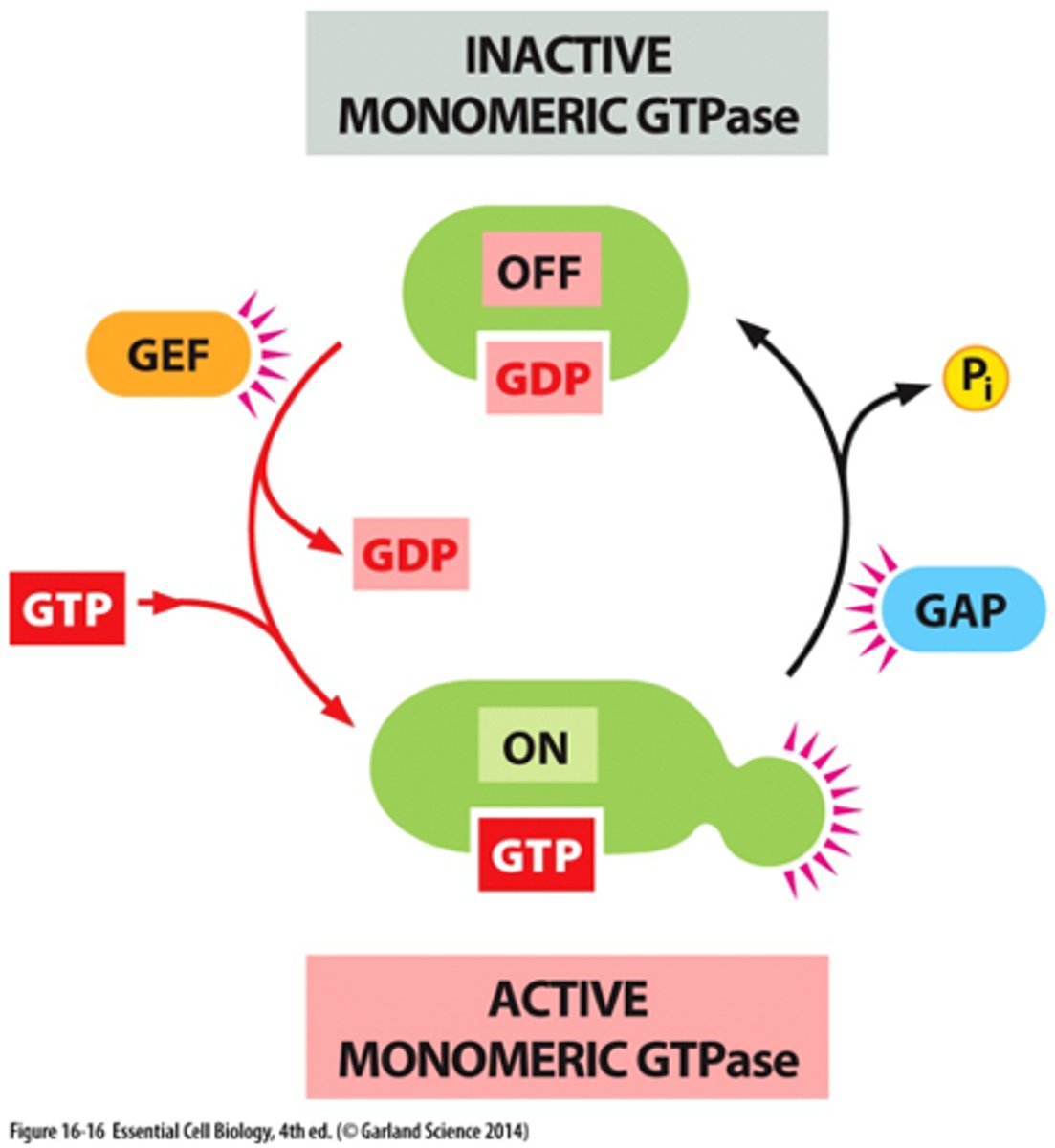
What is the active "on" state GTPase switch proteins?
-activates upon binding GTP, allowing them to interact with and activate downstream effector proteins, propagating the signal further into the cell
-occurs when a guanine nucleotide exchange factor (GEF) replaces GDP with GTP
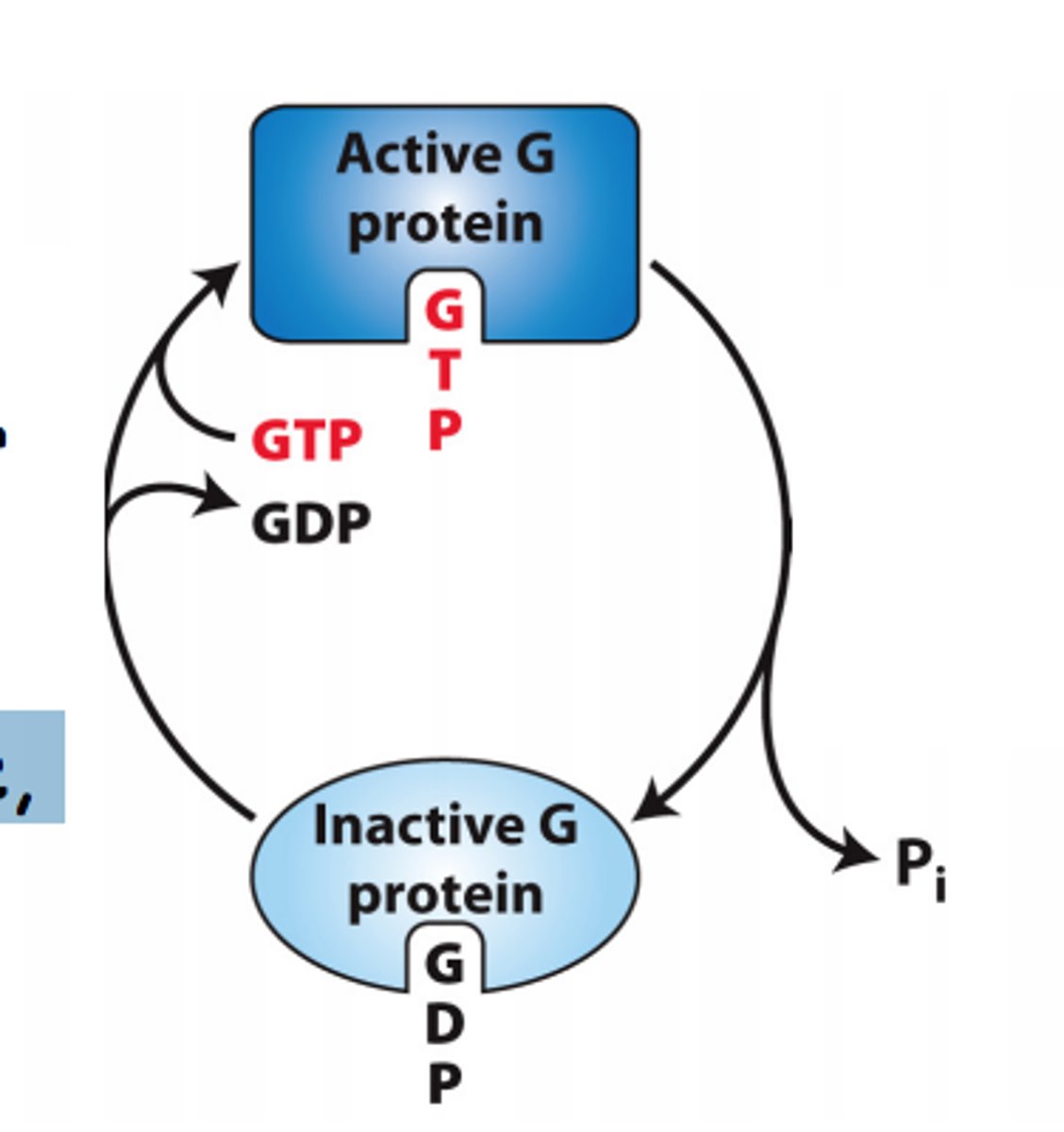
What is the inactive "off' state of GTPase switch proteins?
-bound to GDP (guanosine diphosphate)
-unable to activate downstream signaling proteins
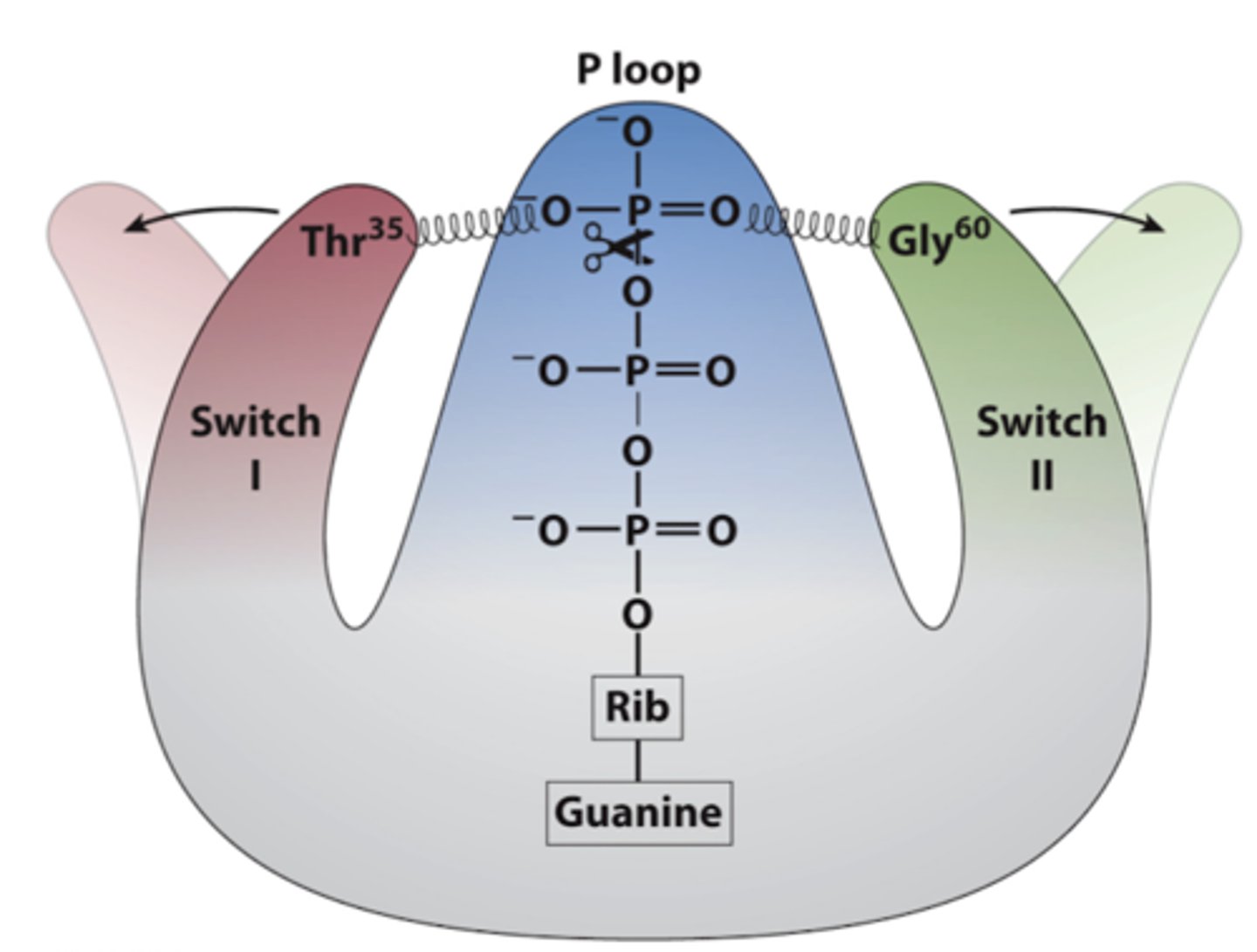
What are the roles of switch I and II in G proteins?
-two regions that change conformation based on whether GTP (active state) or GDP (inactive state) is bound
-When GTP is bound, the switches are "spring-loaded," allowing the G protein to activate downstream signaling pathways
-When GDP is bound, the G protein is inactive and cannot interact with target proteins.
Guanine Nucleotide Exchange Factor (GEF)
-facilitates the exchange of GDP for GTP on GTPase switch proteins
-convert them from inactive state (GDP-bound) to their active state (GTP-bound)
What are second messengers?
-small cytoplasmic signaling molecules produced in response to ligand binding to a receptor, facilitating signal transfer and amplifying cellular responses.
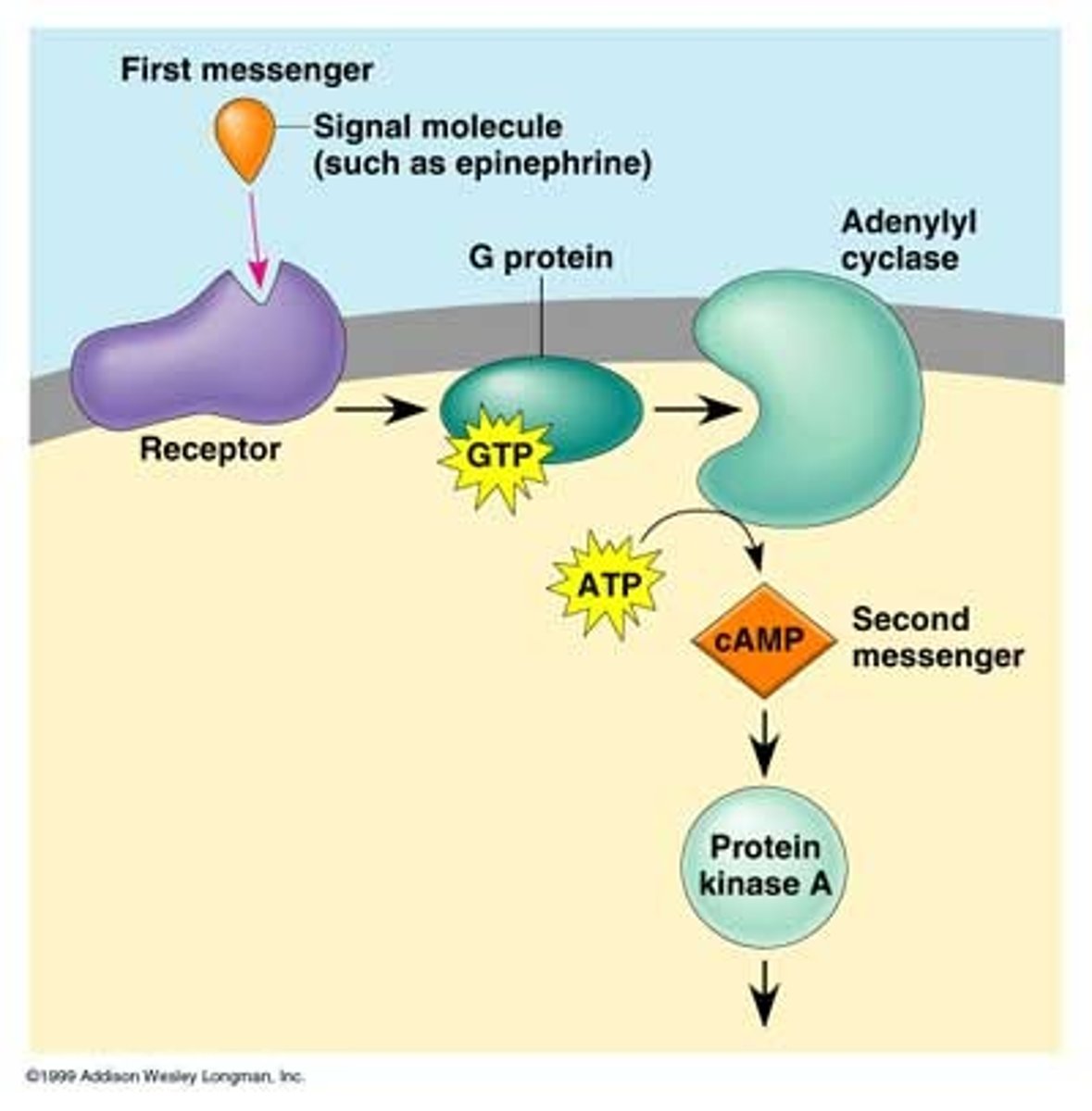
What are the most common second messengers?
-Cyclic AMP (cAMP) and Cyclic GMP (cGMP)
-Diacylglycerol (DAG)
-Inositol trisphosphate (IP3)
-Ca2+ ions
How do second messengers function in signal transduction?
They activate various kinases, open ion channels, and promote signal amplification, allowing for rapid cellular responses to external signals
Why are second messengers important for signal amplification?
Their production can lead to signal amplification, as one activated receptor can generate many second messenger molecules, enhancing the cellular response.
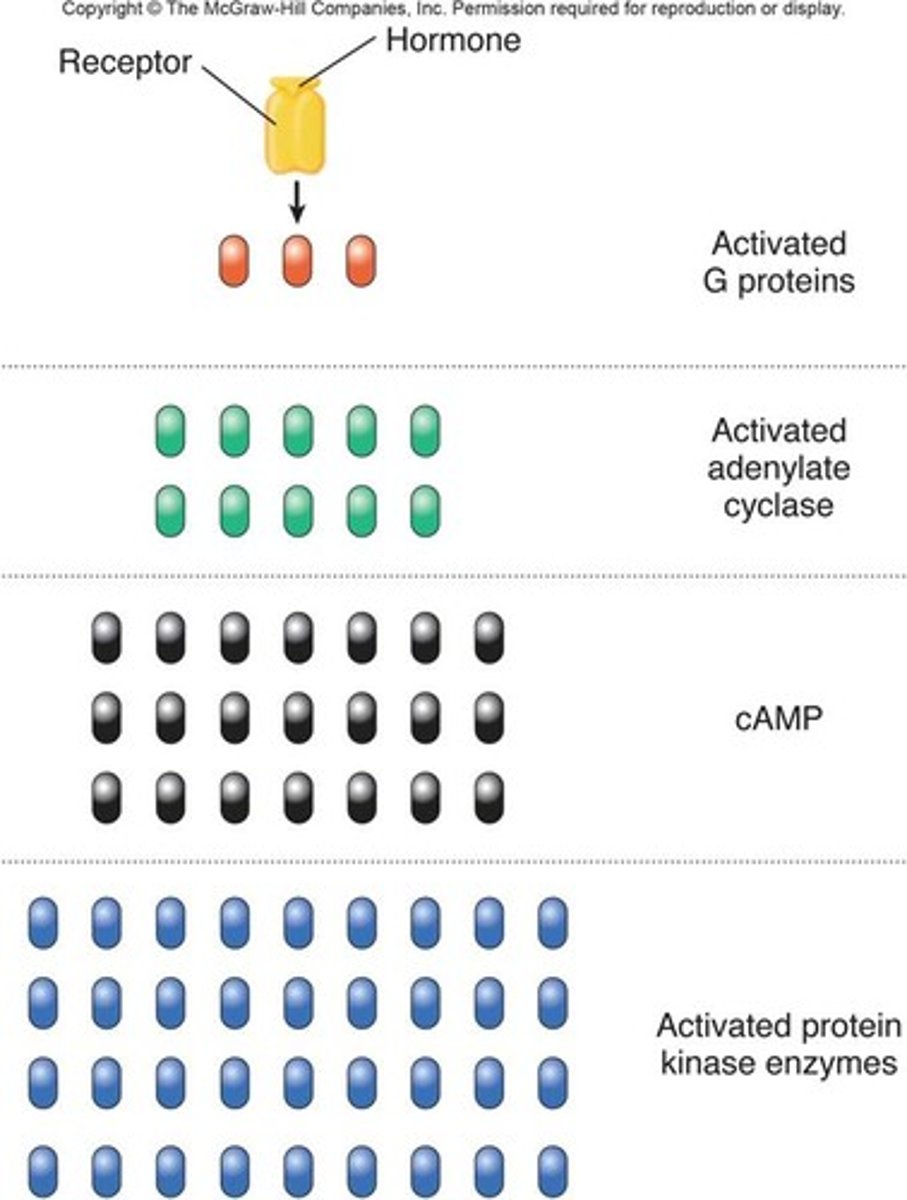
How do second messengers differ from larger signaling proteins?
Second messengers can diffuse quickly within the cytoplasm, allowing for rapid signal transmission, whereas larger signaling proteins are slower to move and act.
How are receptors distributed in the plasma membrane (PM)?
Receptors are typically clustered into discrete regions of the plasma membrane rather than being uniformly distributed, which enhances signaling efficiency.
two major ways receptors are clustered
1) Via cytosolic adapter proteins
2) Within lipid rafts
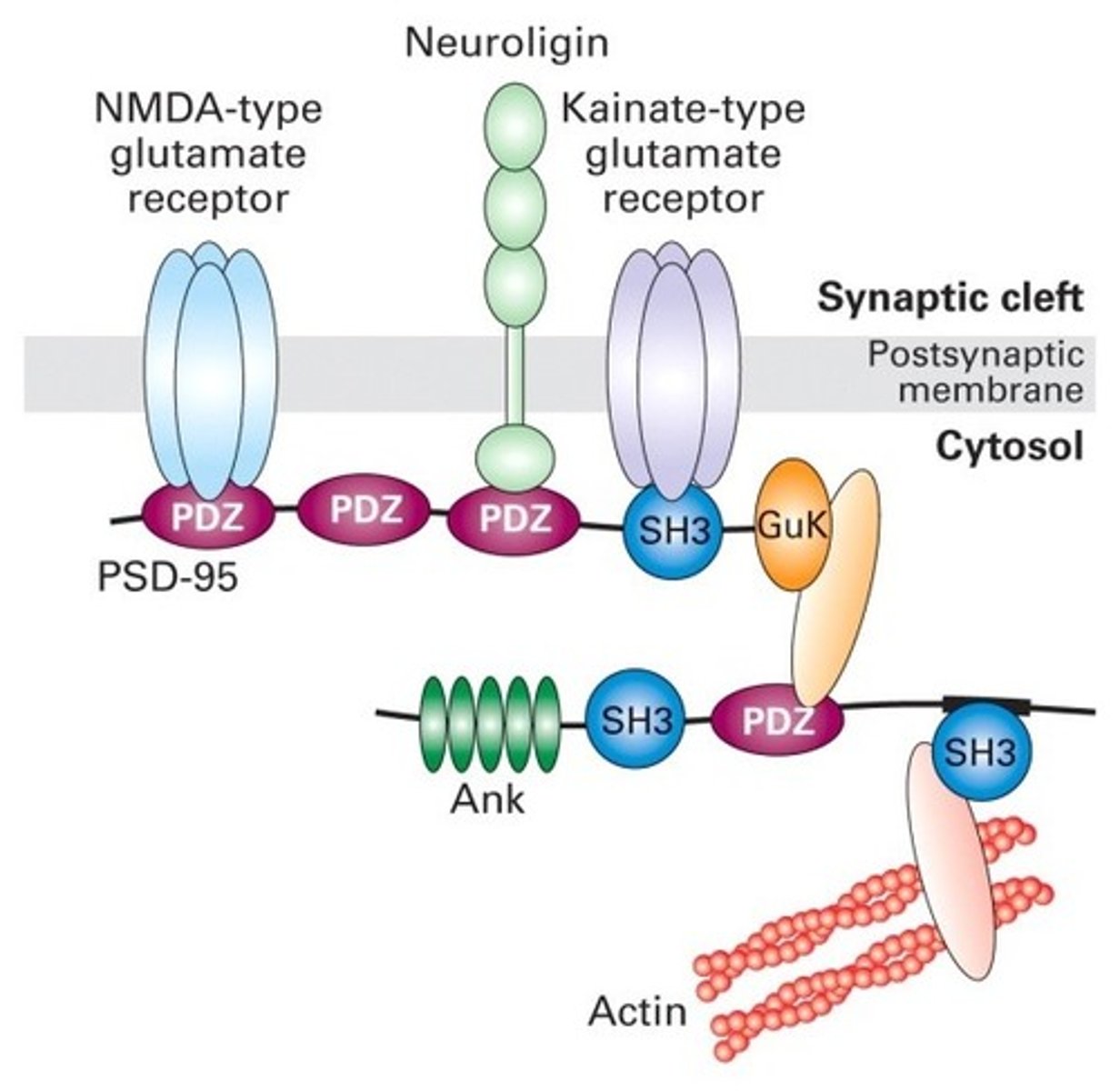
How do cytosolic adapter proteins facilitate receptor clustering?
-contain multiple protein-protein interaction domains
-allows one adapter to indirectly link several membrane receptors together
-ex: PDZ domains are specific interaction domains that allow these proteins to bind with one another
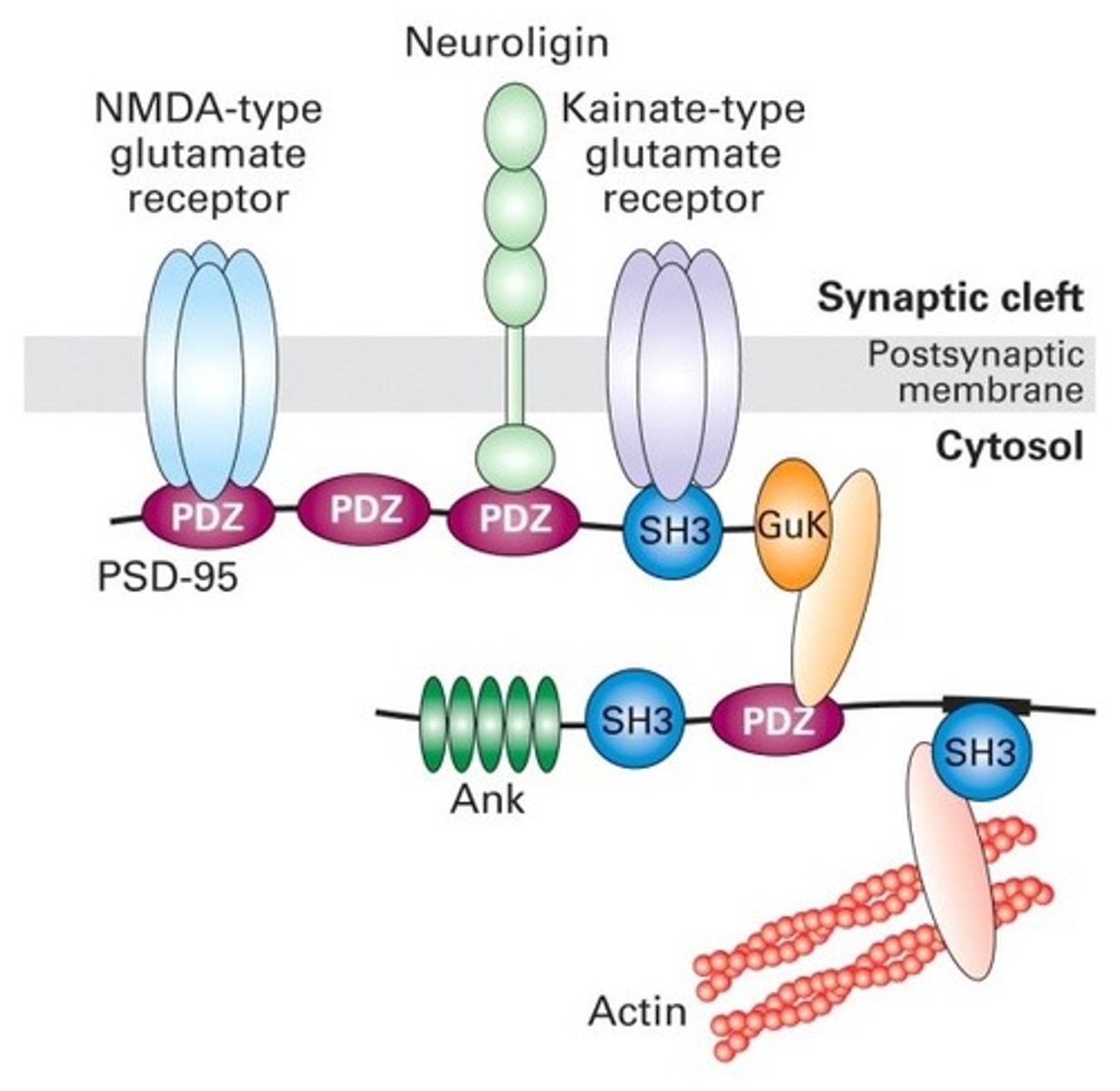
effect of ligand binding on lipid rafts
often signal a receptor to move laterally to a lipid rafts, promoting efficient signaling
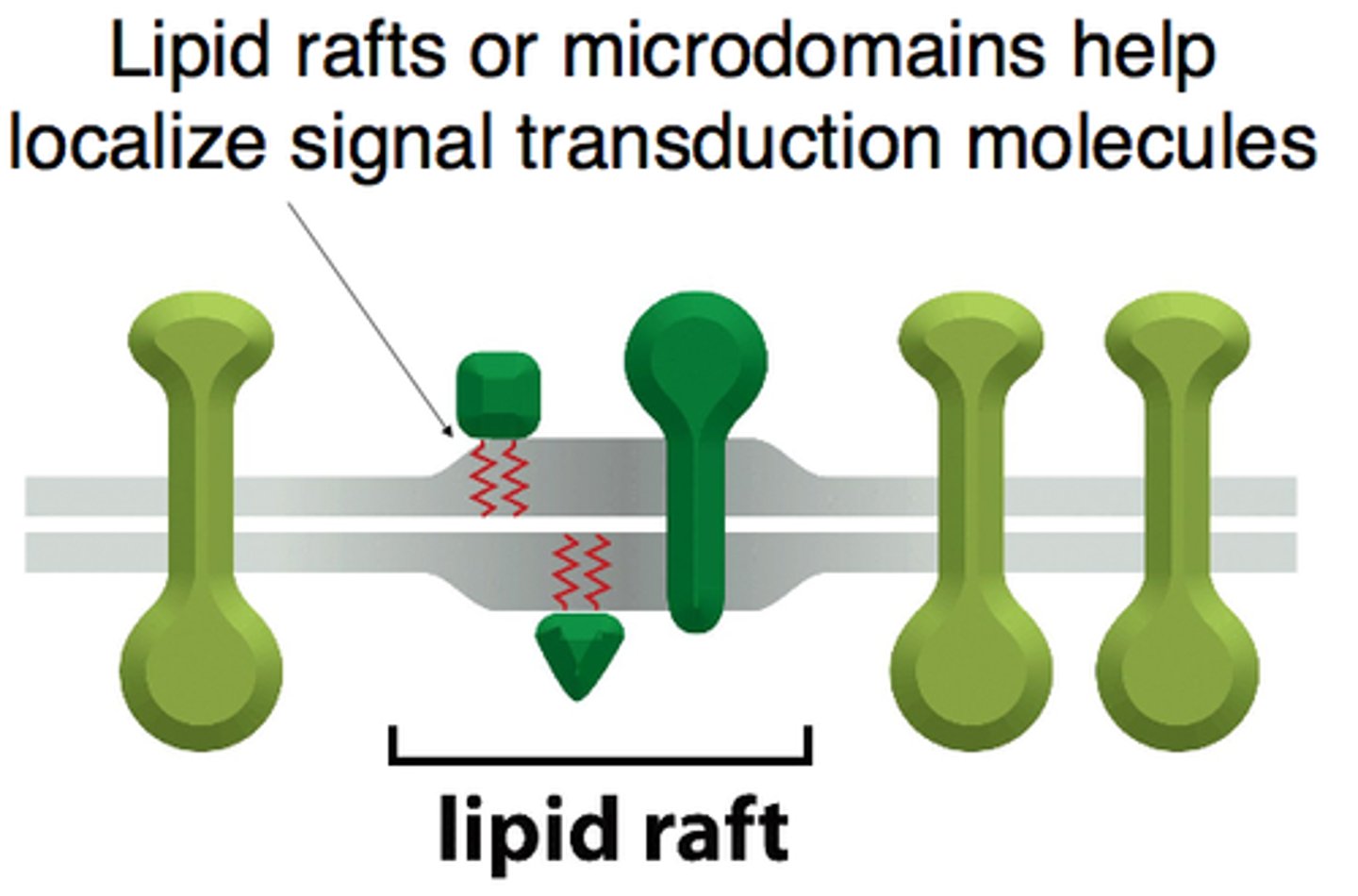
What are lipid rafts and how do they contribute to signaling efficiency?
-specialized microdomains within the plasma membrane that are rich in cholesterol and specific lipids
-serve as organizational hubs for various signaling molecules and receptors.
-clustering of receptors within lipid rafts promotes efficient signaling by bringing receptors and their associated signaling components into close proximity
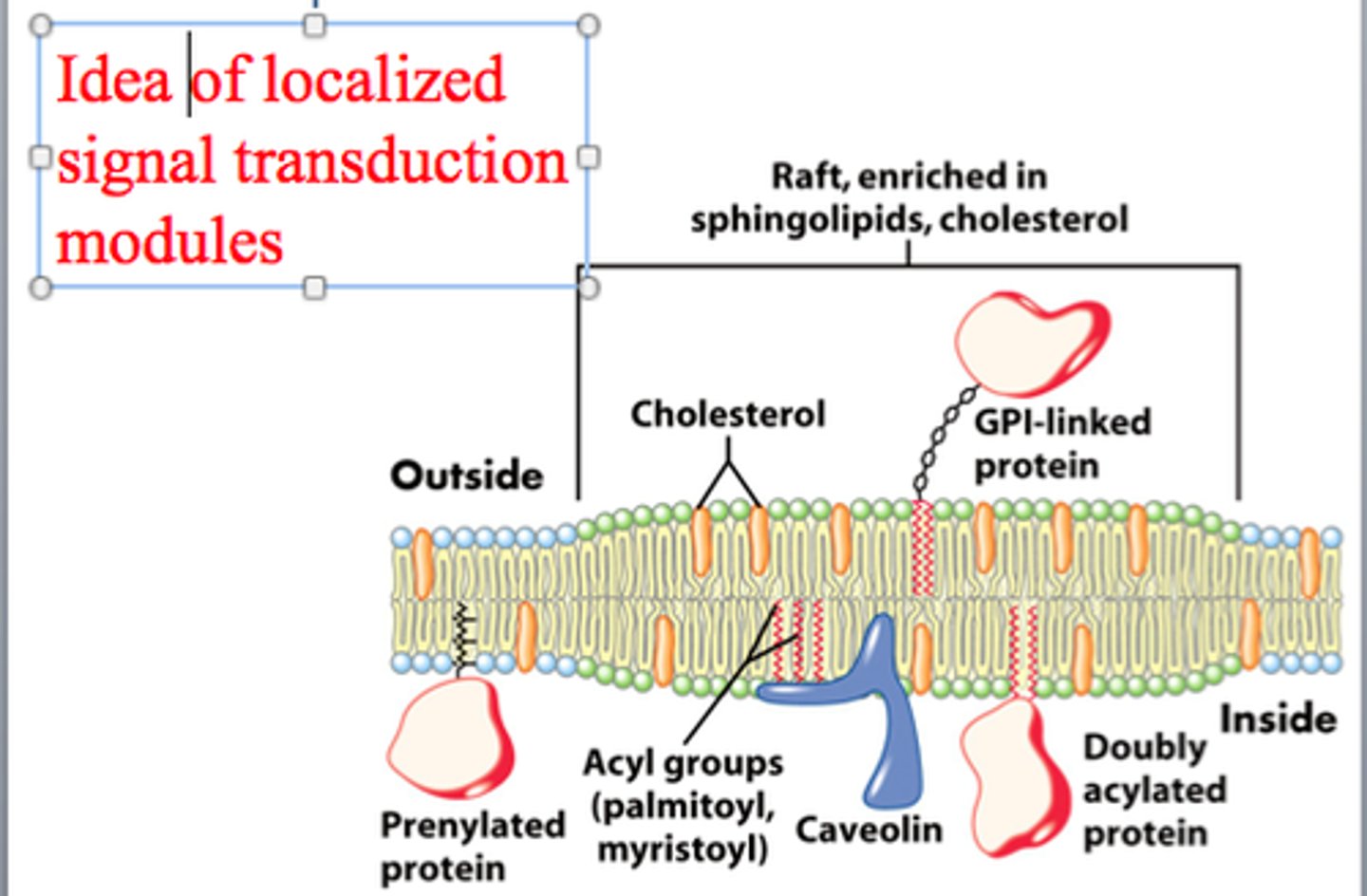
Why is clustering of receptors and signaling components beneficial?
Clustering enhances the efficiency of signaling by bringing receptors and their associated signaling components into close proximity, facilitating rapid and effective cellular responses.
What are G-protein coupled receptors (GPCRs)?
-large family of receptors characterized by their 7 membrane-spanning regions
-play a crucial role in transmitting signals from outside the cell to the inside.
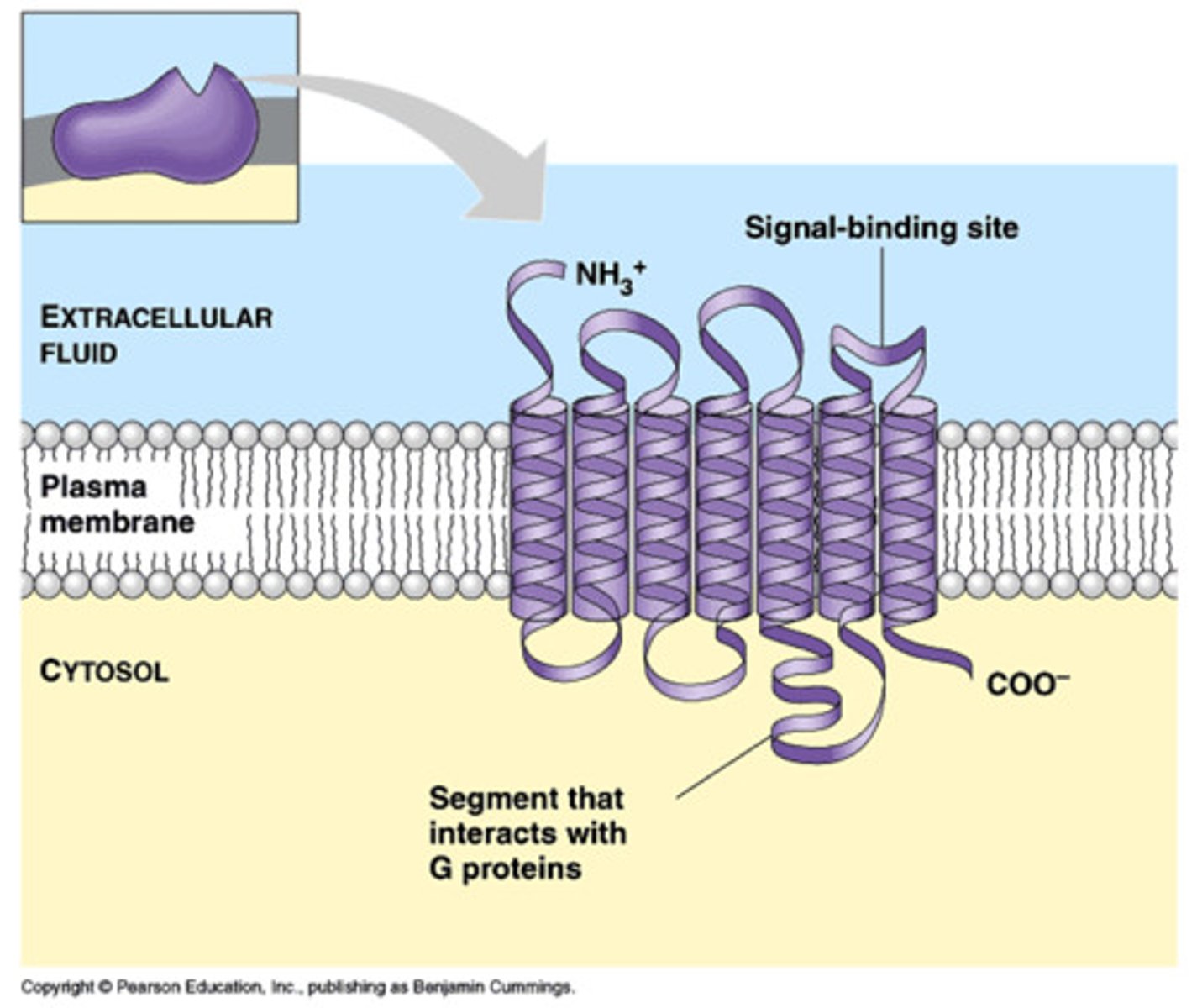
How do G proteins interact with GPCRs?
interact with the cytosolic loop 3 and the C-terminus of GPCRs, facilitating the transmission of signals initiated by ligand binding
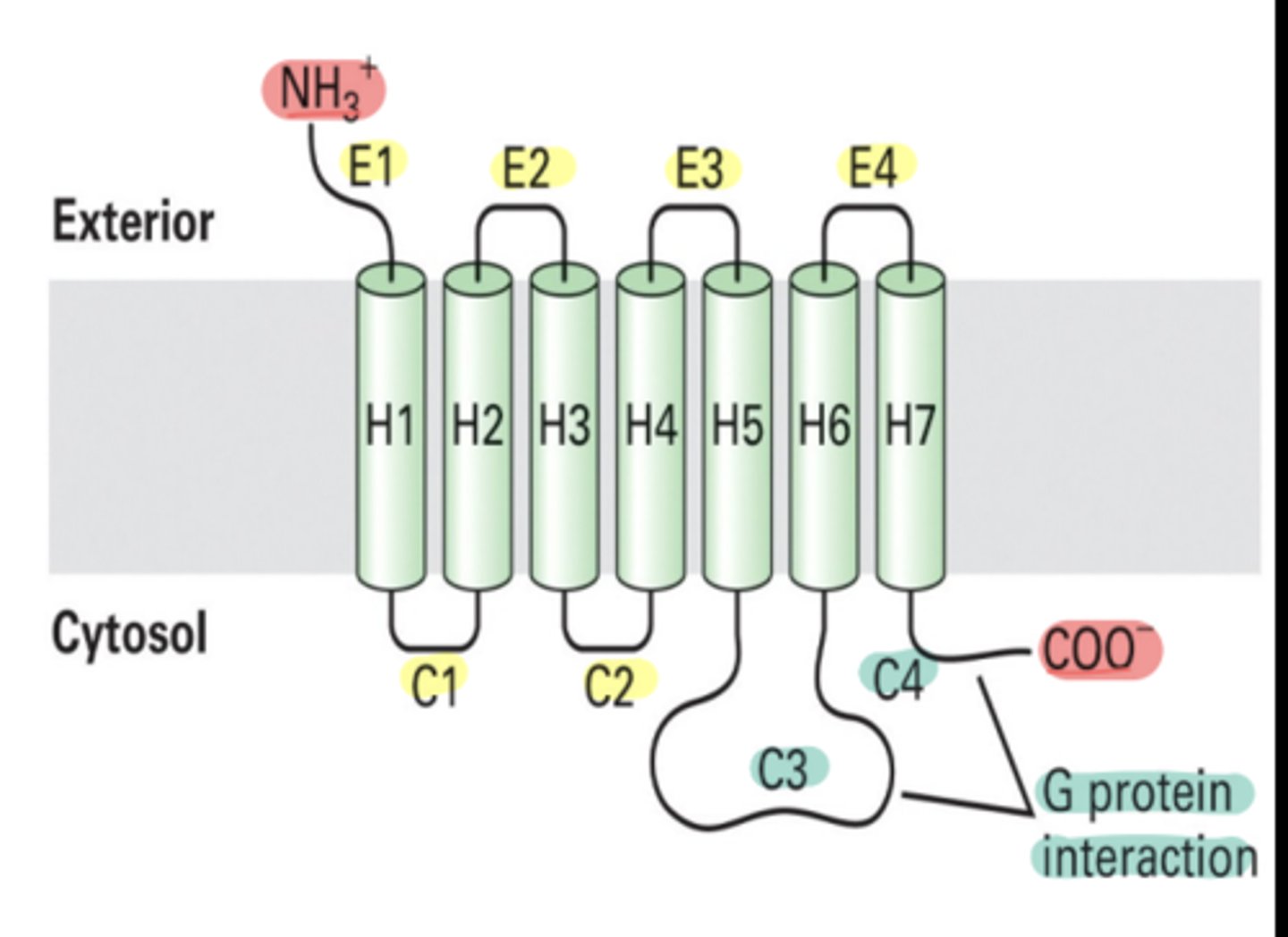
What types of signals do GPCRs respond to?
respond to a variety of signals, including hormones (e.g., epinephrine), neurotransmitters, light-activated signals in the eye (e.g., rhodopsin), and smell receptors in the nose
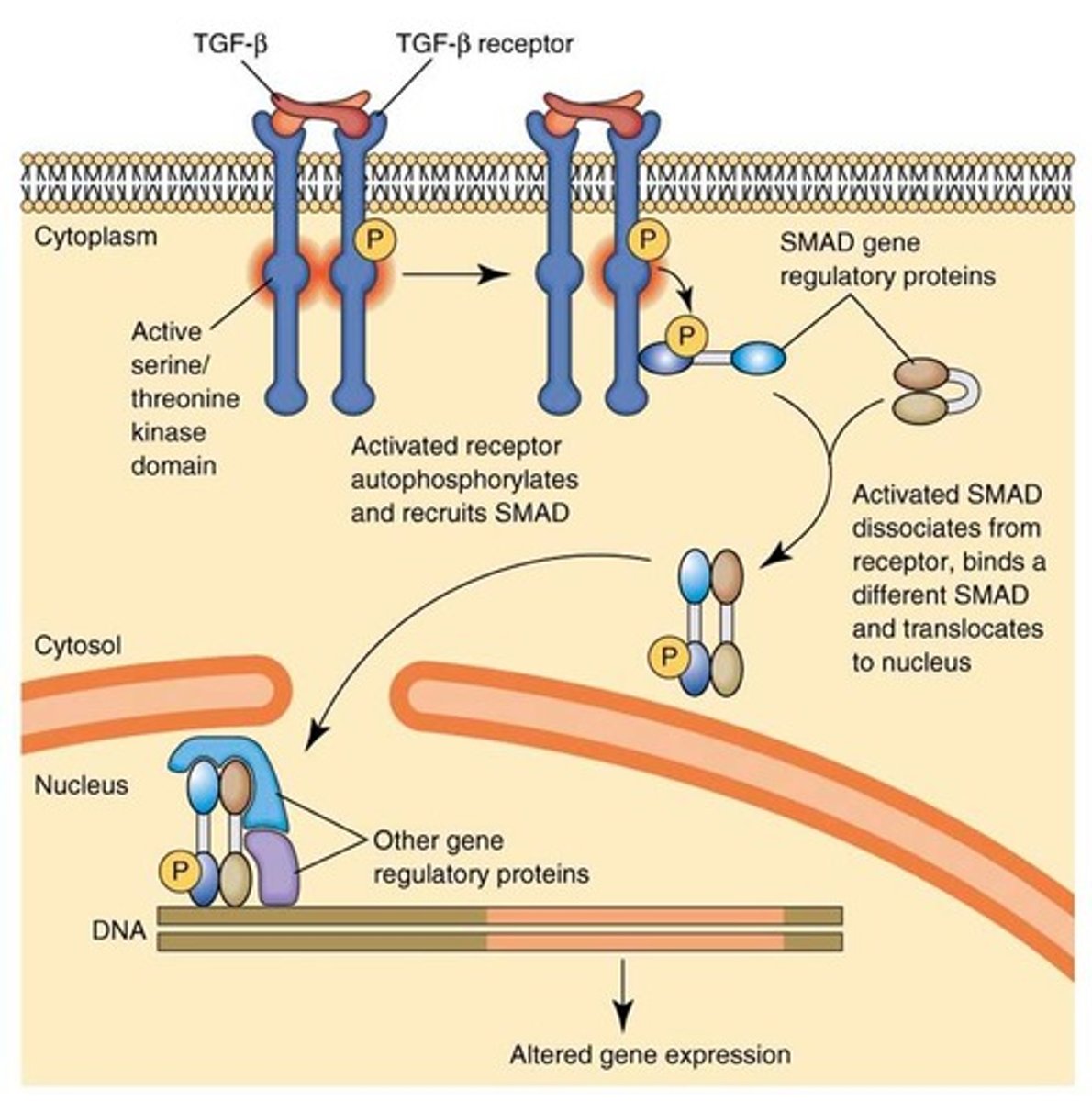
What are the steps in a simple signal transduction pathway involving a receptor, kinase, and transcription factor?
1. receptor is bound to an inactive protein kinase when no ligand is present
2. ligand binds to the receptor -> conformational change
3. activated receptor -> activation of the attached kinase
4. kinase phosphorylates an inactive transcription factor, causing it to dimerize.
5. dimerized transcription factor moves into the nucleus
6. transcription factor activates the transcription of target genes
7. phosphatase removes the phosphate group, reverting the transcription factor to its inactive state, which then moves back to the cytosol
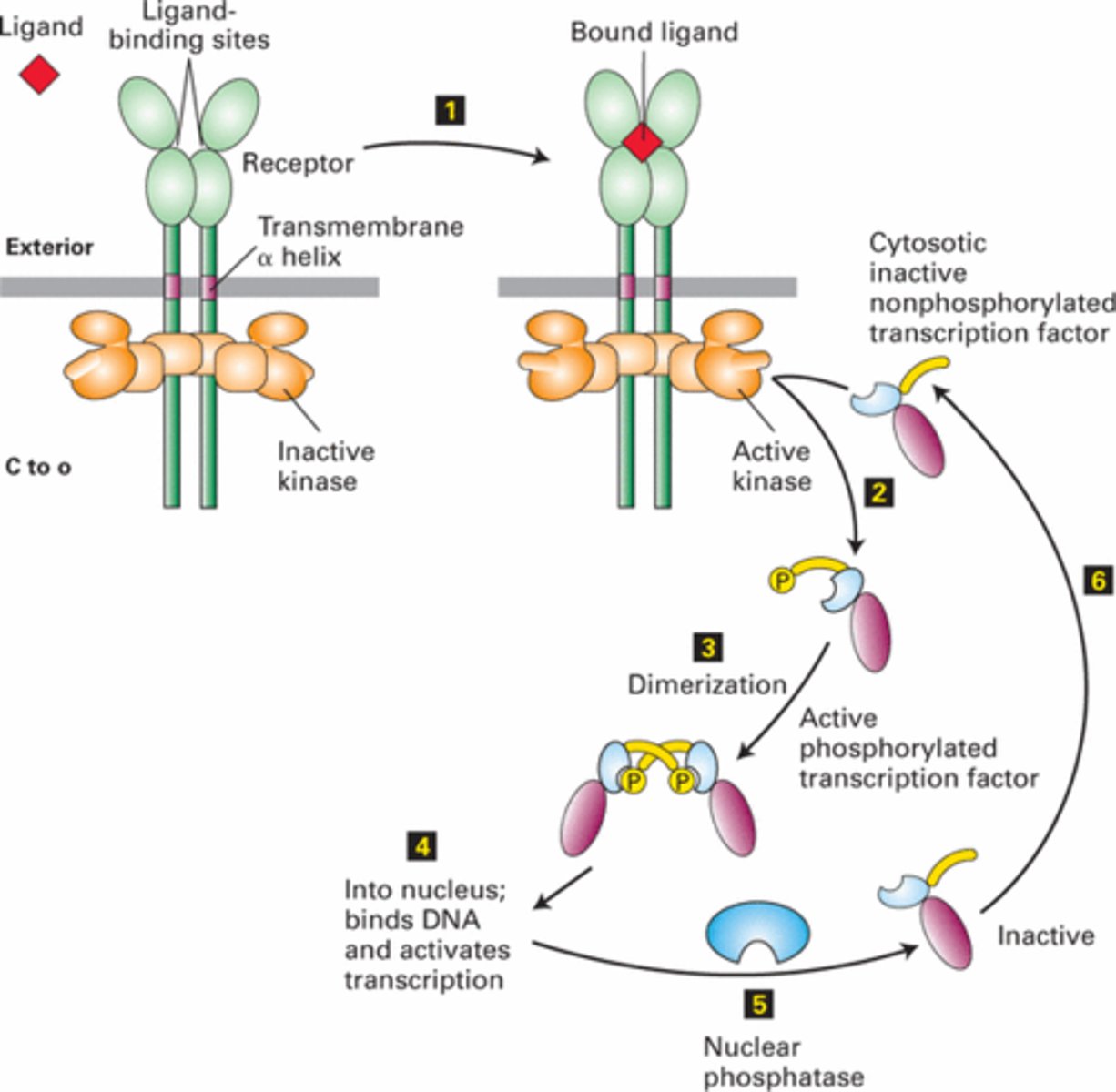
What is a transcription factor, and how is it involved in signal transduction?
a protein that, when phosphorylated and activated, can enter the nucleus to promote the transcription of specific genes, influencing cellular functions.
How is the transcription factor deactivated in a signal transduction pathway?
A phosphatase removes the phosphate group from the transcription factor, returning it to its inactive monomer form, which then moves back to the cytosol.
receptor-associated kinases
-Many receptors have cytosolic (inside the cell) regions that either contain their own protein kinase domains or are closely associated with cytosolic kinases.
-include Protein Kinase Domains - segments within the receptor that can add phosphate groups (phosphorylate) to other proteins
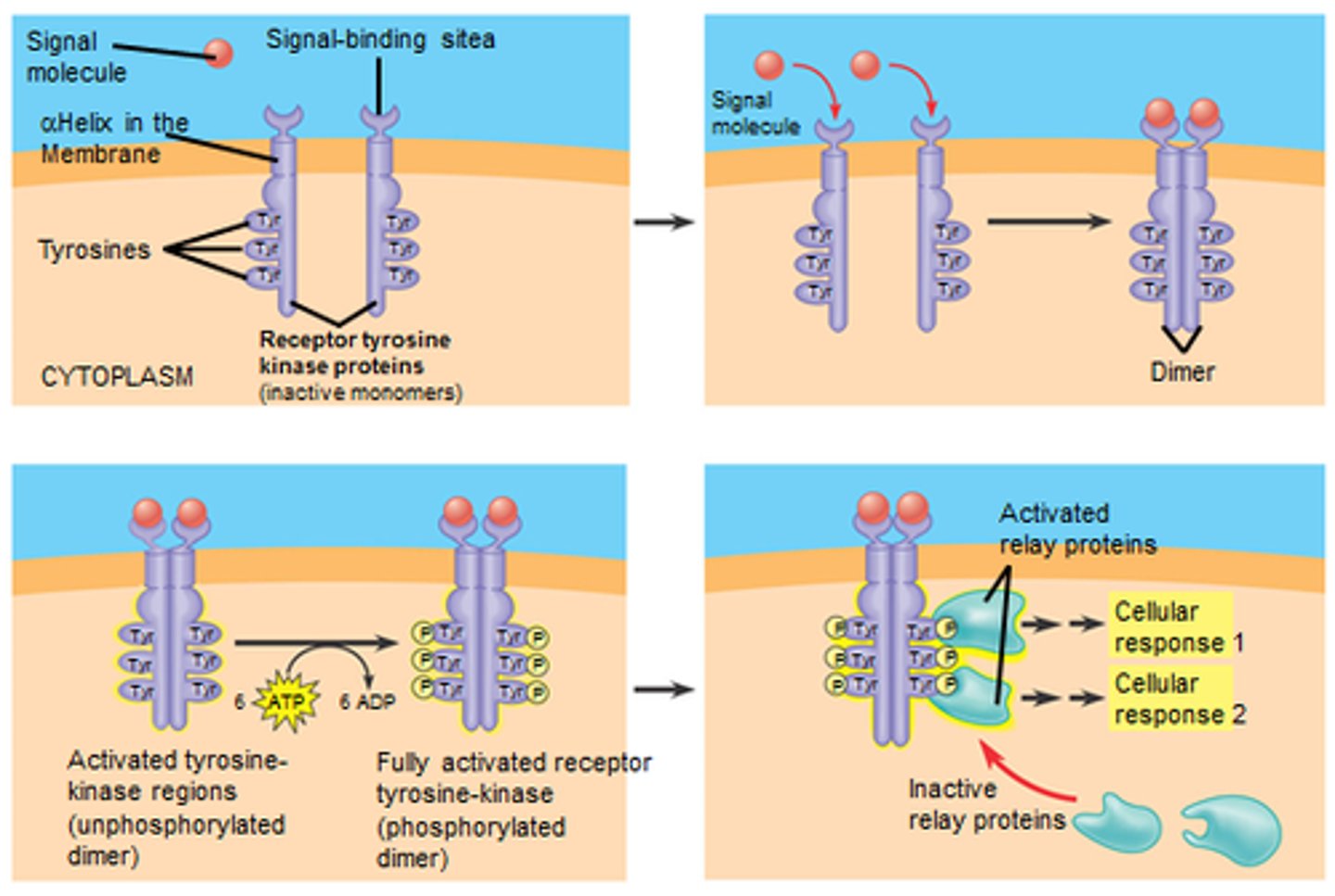
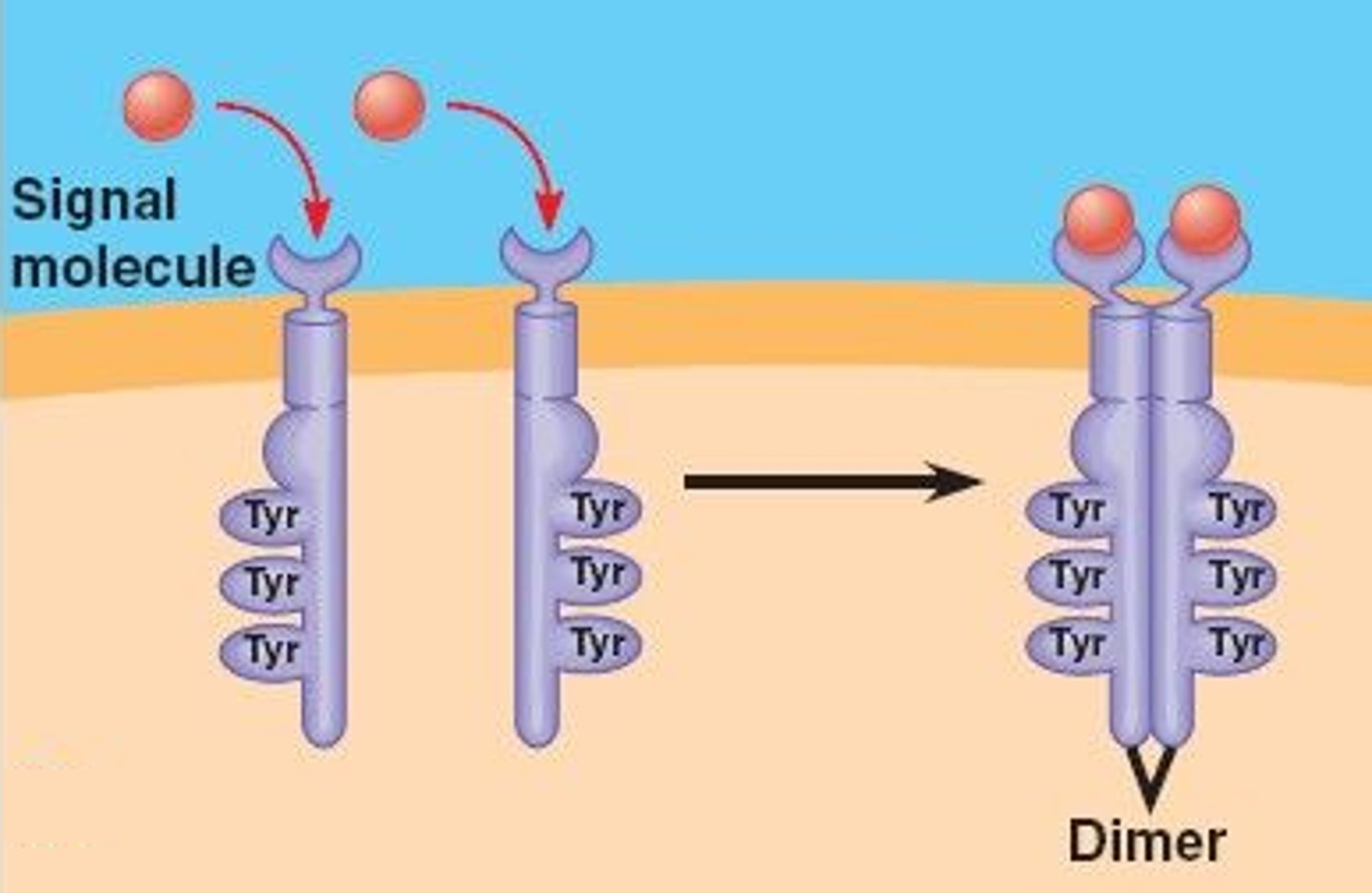
Receptor Dimerization
-process involves two receptor molecules coming together to form a dimer (a complex of two identical molecules)
-common mechanism for activating receptor associated kinases
What role do small GTP-binding switch proteins like Ras play in signal transduction?
-Ras is activated by receptor-associated kinases and initiates multiple kinase cascades, where one kinase phosphorylates and activates another
-This can lead to various cellular responses, including the activation of transcription factors.
How do cytosolic kinases function in signal transduction pathways?
-seven-spanning receptors activate larger GTP-binding Gα proteins, which in turn activate specific kinases or other signaling proteins, playing a crucial role in transmitting signals from the receptor to intracellular targets
What happens during protein subunit dissociation in signaling pathways?
-In several pathways, the disassembly of multiprotein complexes occurs in the cytosol, releasing a transcription factor that translocates into the nucleus to exert its effects on gene transcription
-crucial part of the signaling pathways that regulate inflammation
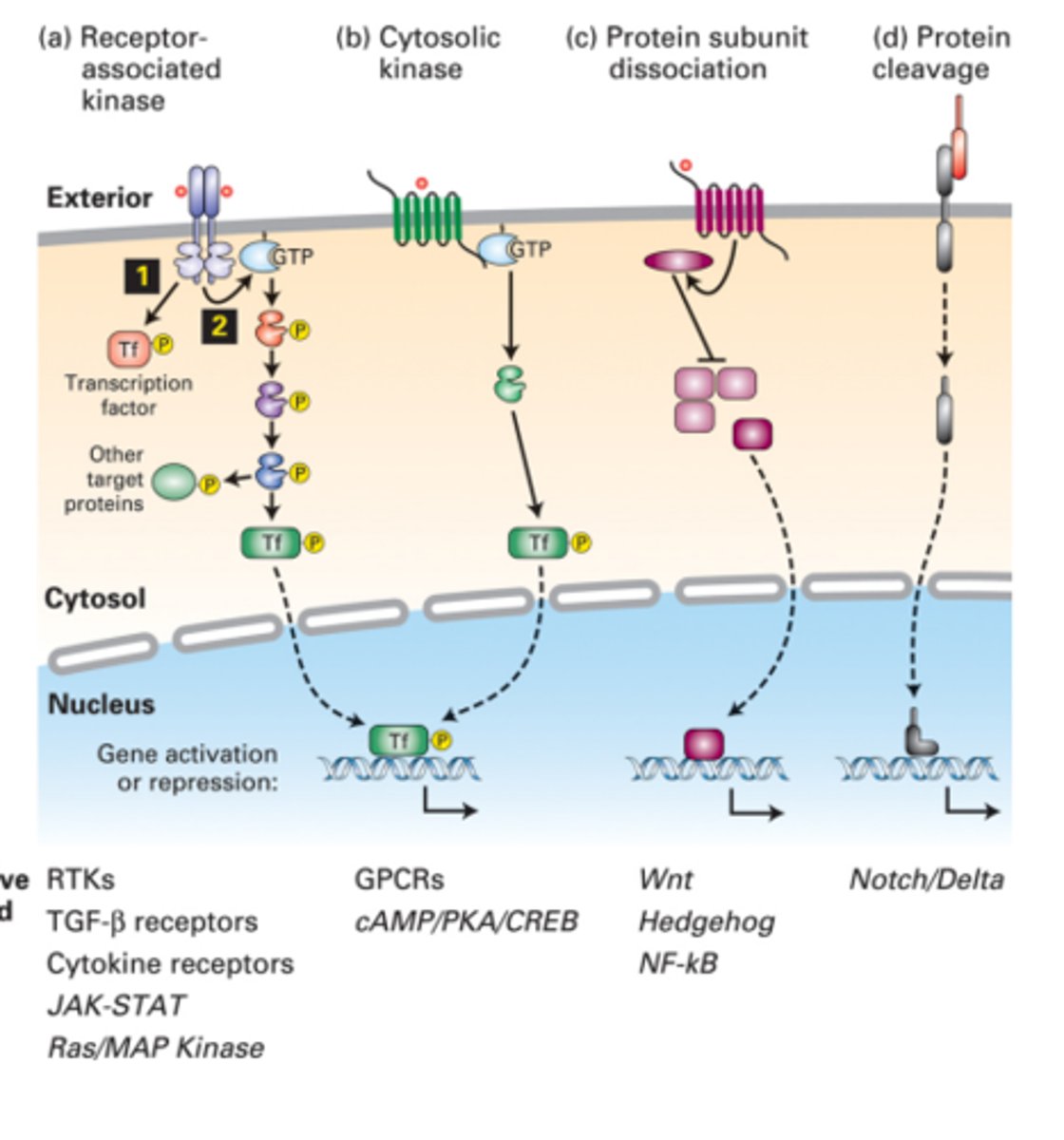
What is the significance of protein cleavage in some signaling pathways?
-In certain signaling pathways, proteolytic cleavage of a receptor can release an active transcription factor.
-This process is often irreversible and leads to permanent changes in gene expression
What are the effects of ligand binding on existing proteins?
Ligand binding activates G proteins, leading to activation of kinases and opening of ion channels, resulting in short-term, rapid responses
How do ligand-receptor interactions affect gene transcription?
-can activate signaling pathways that lead to the activation or inhibition of gene transcription, resulting in new protein production
-ex: TNFa activates transcription of over 150 genes
What are the basic steps of signal transduction leading to transcription?
1. Ligand binds to receptor, activating it
2. Activated receptor activates a nearby cytosolic protein which activates another protein
3. One or more transcription factors are activated or repressed, altering transcription.
Why is it vital to understand signaling pathways?
-These signals ultimately decide whether a cell will live or die, divide or stop dividing, stay undifferentiated or differentiate, stay normal or develop into a tumor
-Understanding these pathways helps in grasping concepts related to cancer, aging, cell regeneration after injury, and immune system disorders, providing targets for treatment.
-targets for treatments!!!
Cytokine receptors and JAK/STAT signaling
Promote cell proliferation and differentiation
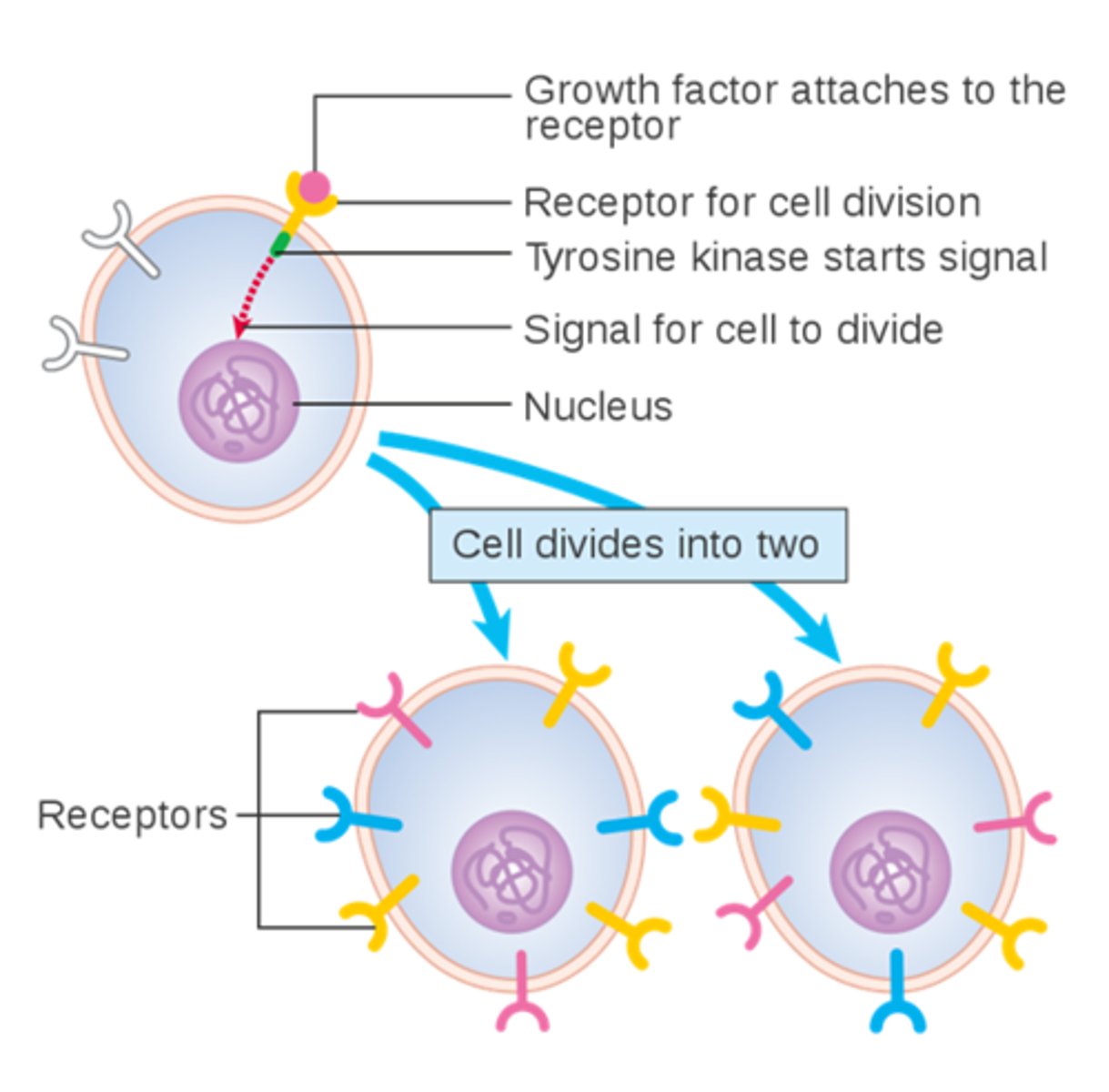
Receptor tyrosine kinases and Ras/MAPK signaling
Modulate growth, proliferation, differentiation, and survival.
NFκB activation
Regulates immunity

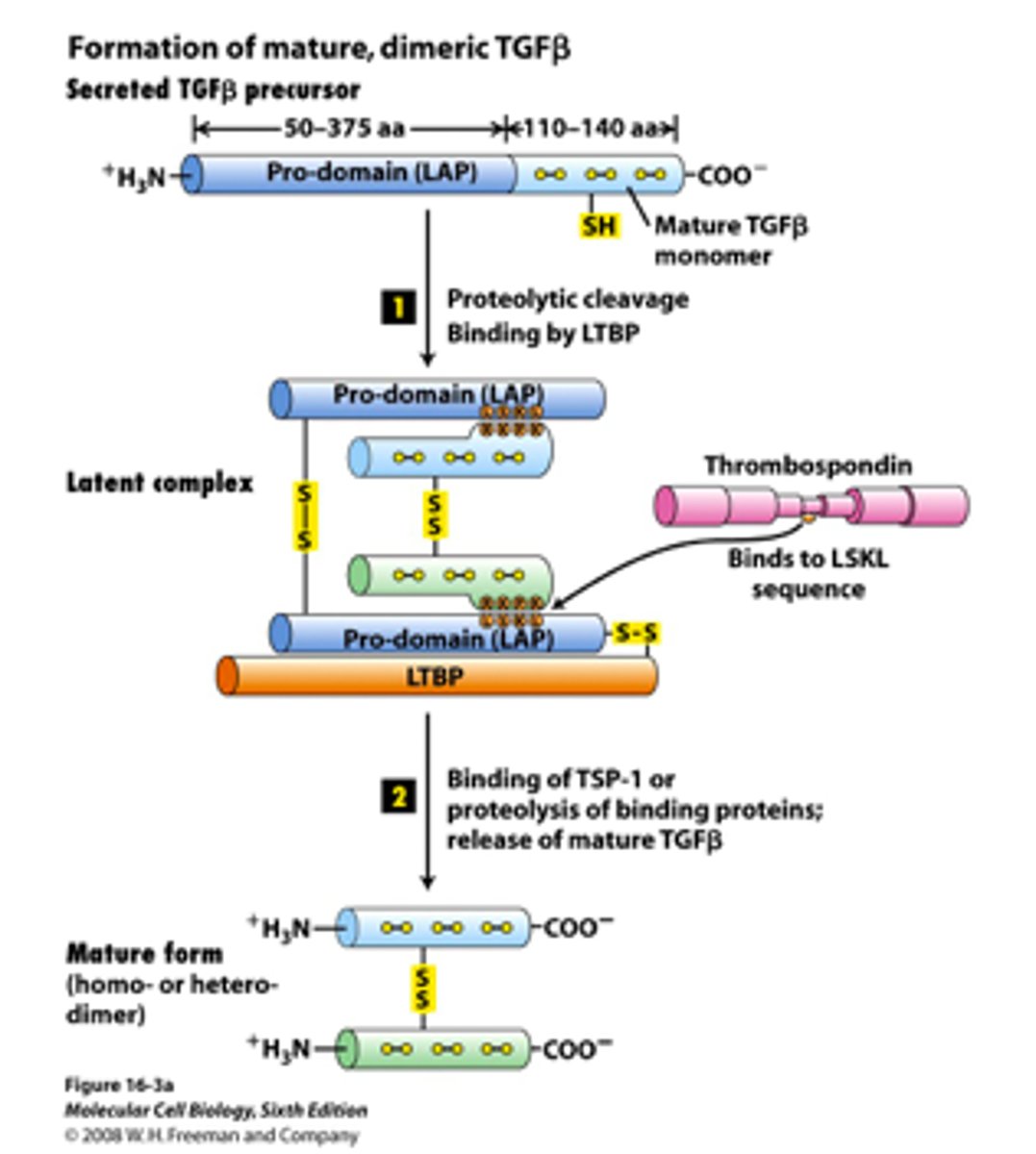
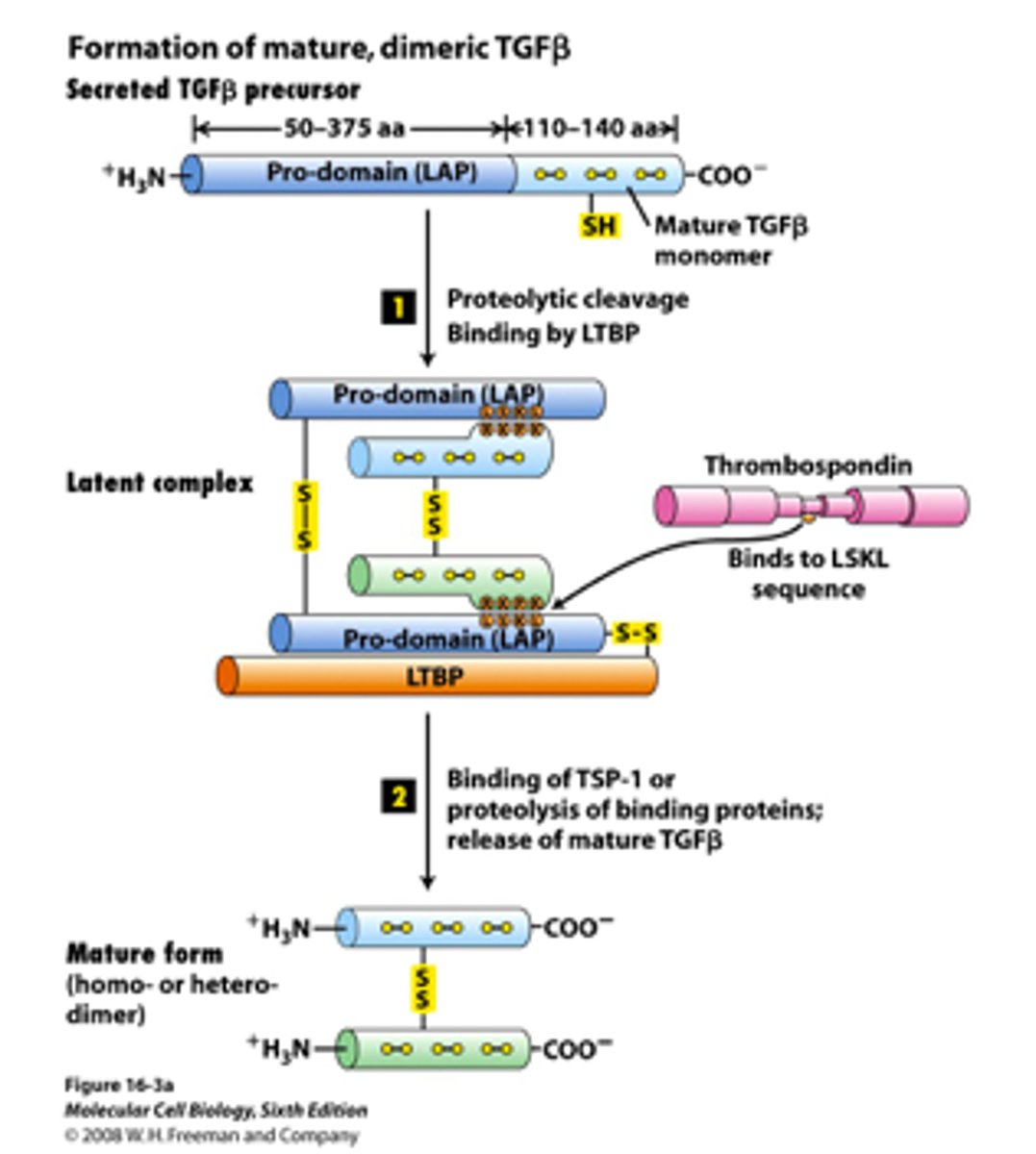
Overview of Steps Involved in Formation of Mature, Dimeric TGFβ
1. TGFβ is released from the cell in an inactive form, consisting of a mature domain and a pro-domain
2. pro-domain recruits Latent TGFβ Binding Protein (LTBP), forming an inactive complex
3. Changes in extracellular matrix (ECM) proteins induce a conformational change in the LTBP-TGFβ complex
4. mature TGFβ domain is released from the pro-domain, and LTBP is degraded.
5. released mature TGFβ molecules dimerize to form active TGFβ, which is ready to interact with TGFβ receptors
What is TGFβ and what effects does it have on cells?
-Transforming growth factors beta
-family of growth factors that influence cell division, differentiation, death, and angiogenesis
-leads to changes in expression of SEVERAL HUNDRED genes
Which cells can produce and respond to TGFβ?
Almost all animal cells can produce and respond to TGFβ, as all cell types have receptors for it.
What is the general effect of TGFβ on cell proliferation and differentiation?
-inhibitor of cell proliferation and differentiation
-typically causes cells to stop in the G1 phase of the cell cycle (aka cell cycle arrest)
What form is TGFβ released from the cell?
inactive form, consisting of a mature domain and a pro-domain.
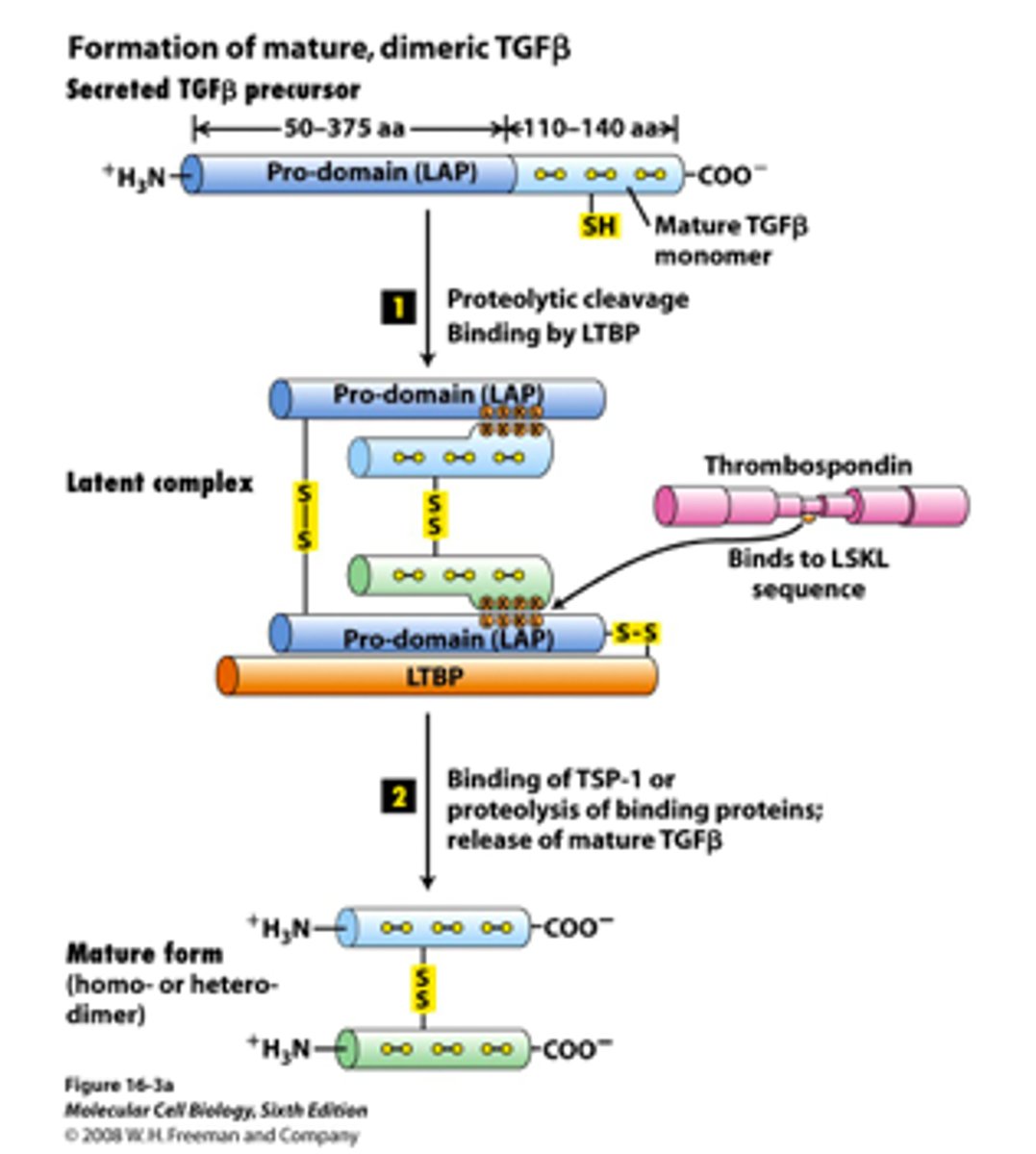
What role does the pro-domain play in TGFβ's inactive form?
The pro-domain remains non-covalently attached to the mature domain and recruits latent TGFβ binding protein (LTBP), forming an inactive complex.
Where does the inactive TGFβ complex typically reside?
The inactive TGFβ complex hangs out in the extracellular matrix (ECM).
What triggers the activation of TGFβ?
Changes in ECM proteins cause a conformational change in the TGFβ complex.
What happens to the pro-domain and LTBP during TGFβ activation?
The pro-domain is released from the mature domain, and LTBP is degraded.
In what form is TGFβ active after the activation process?
Active TGFβ is a dimer, consisting of two mature TGFβ molecules and can now interact with various TGFβ receptors on target cells.
3 main types of TGFβ receptors
R1, RII, and RIII
TGFβ Receptor Activation and Signal Transduction Overview
1. TGFβ released in an inactive form & pro-domain gets cleaved
2. Active TGFβ (as a dimer) binds to TGFβ receptors (RII or RIII)
3. TGFβ binding to RII leads to the recruitment and activation of RI (RIII can bind TGFβ and present it to RII)
4. RII phosphorylates RI, activating its kinase activity.
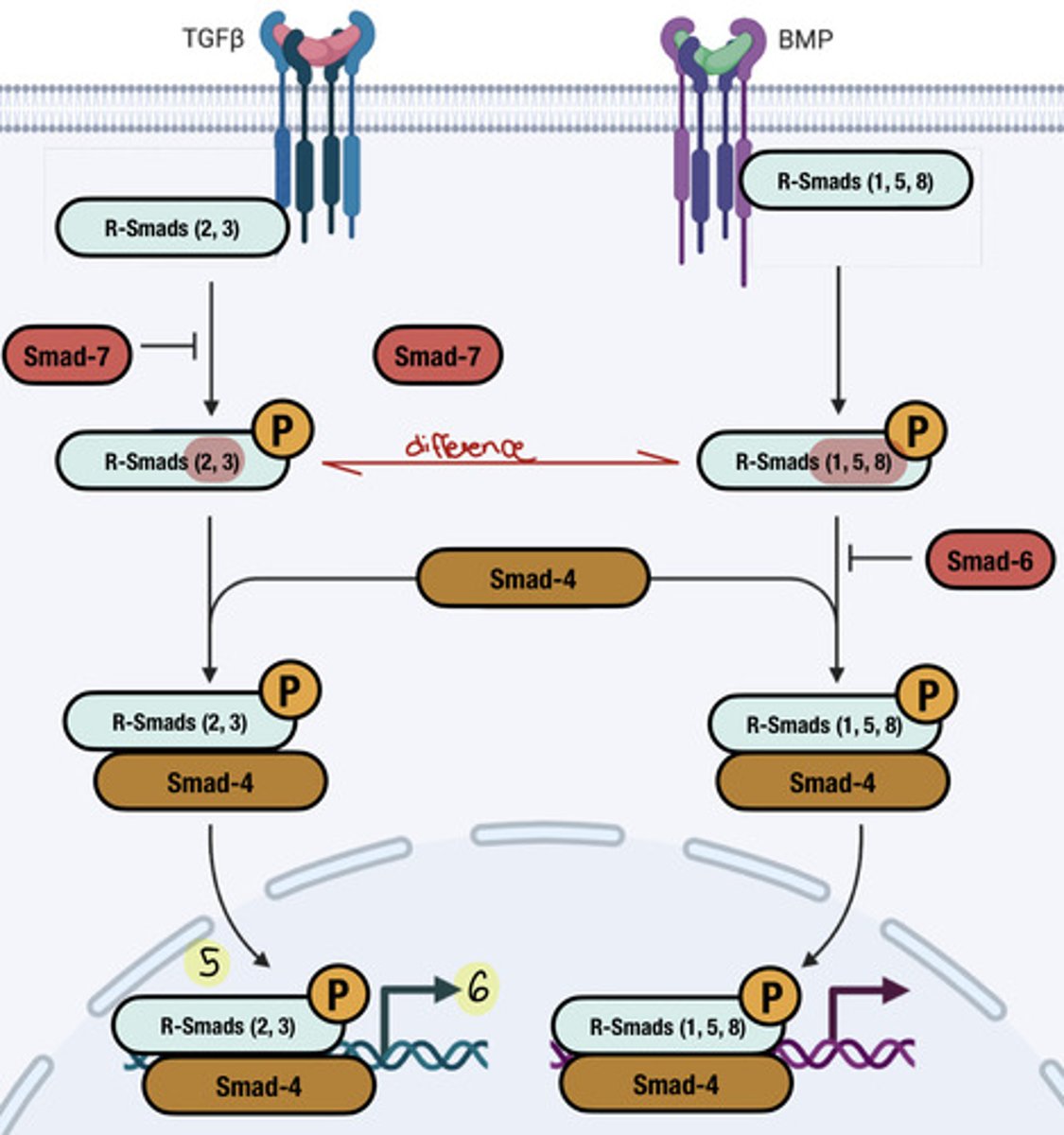
5. Activated RI phosphorylates R-Smads, leading to a conformational change that exposes their nuclear localization signal (NLS)
6. Phosphorylated R-Smads and co-Smad (Smad 4) form a complex with importin and translocate to the nucleus
7. In the nucleus, the R-Smad/co-Smad complex binds to DNA and regulates the transcription of target genes, influencing cell proliferation, differentiation, and other processes.
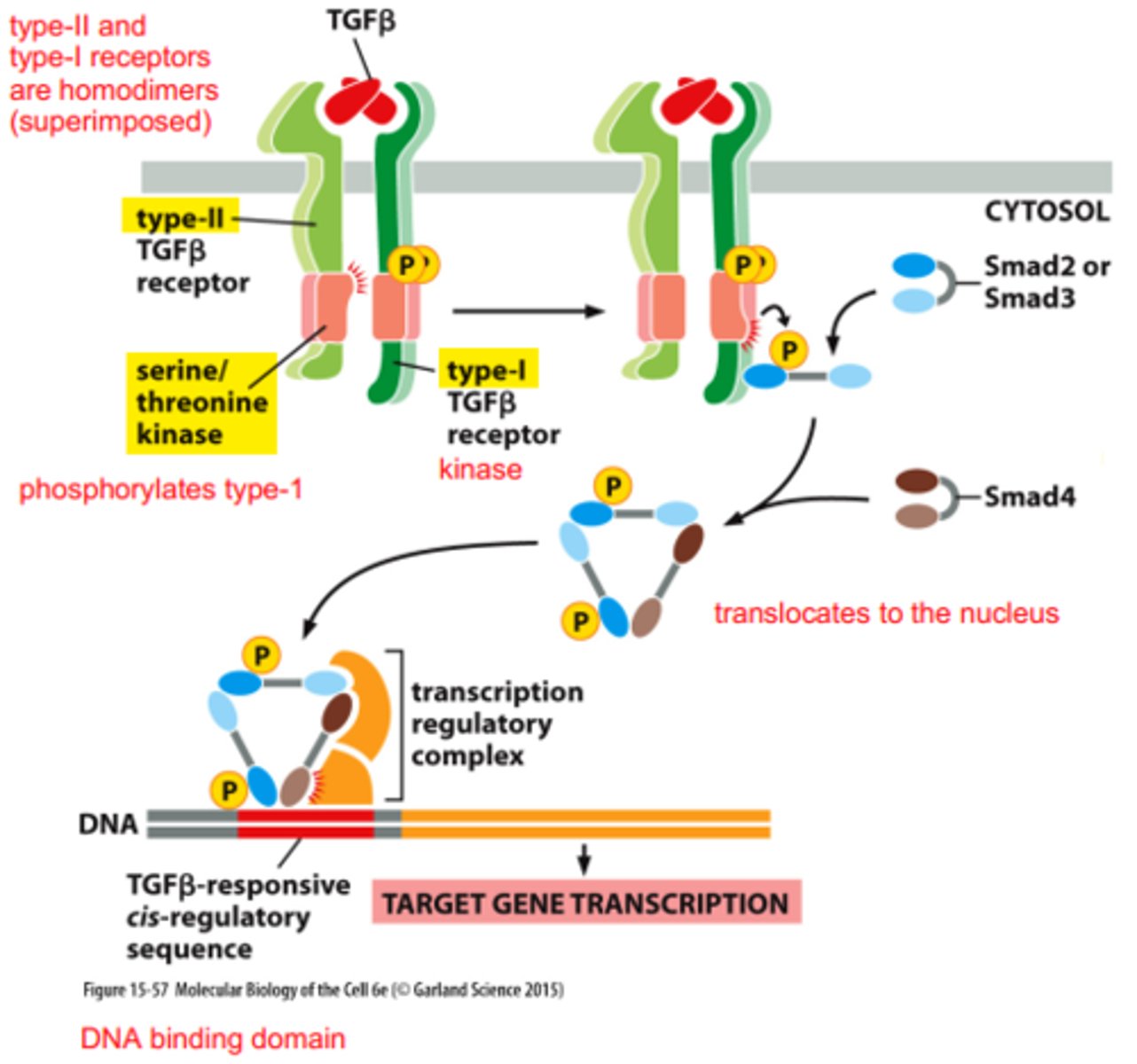
role of the RI receptor in TGFβ signaling
-Does not bind TGFβ directly
-relies on RII and RIII for TGFβ binding
-Required for activation of the signaling pathway.
role of the RII receptor in TGFβ signaling
-Constantly phosphorylated but cannot activate on its own
-Activation occurs upon binding of TGFβ, leading to recruitment and activation of RI
role of the RIII receptor in TGFβ signaling
Can bind TGFβ and present it to RII.
Functions similarly to RII in the signaling process.
What initiates the TGFβ receptor signaling pathway?
Active TGFβ binds to either RII or RIII -> RII forms a complex with RI -> RII phosphorylates RI -> RI undergoes conformation change and has activated kinase ability
What is the function of R-Smads in TGFβ signaling?
-group of transcription factors
-phosphorylated by RI, leading to a conformational change that activates their function in gene transcription
effects of R1 phosphorylating R-Smads
-unmasks their nuclear localization signal (NLS), allowing them to translocate to the nucleus with co-Smad
(simple words: specific sequence or region within a protein, which directs the protein to the nucleus, becomes accessible or active after a conformational change)
effect of unmasked NLS on phosphorylated R-smads
Two phosphorylated R-Smads form a complex with co-Smad (Smad 4 and importin), which goes to the nucleus and regulates gene transcription
cytokines
family of small, secrete proteins that generally help PROMOTE cell differentiation, growth, and proliferation
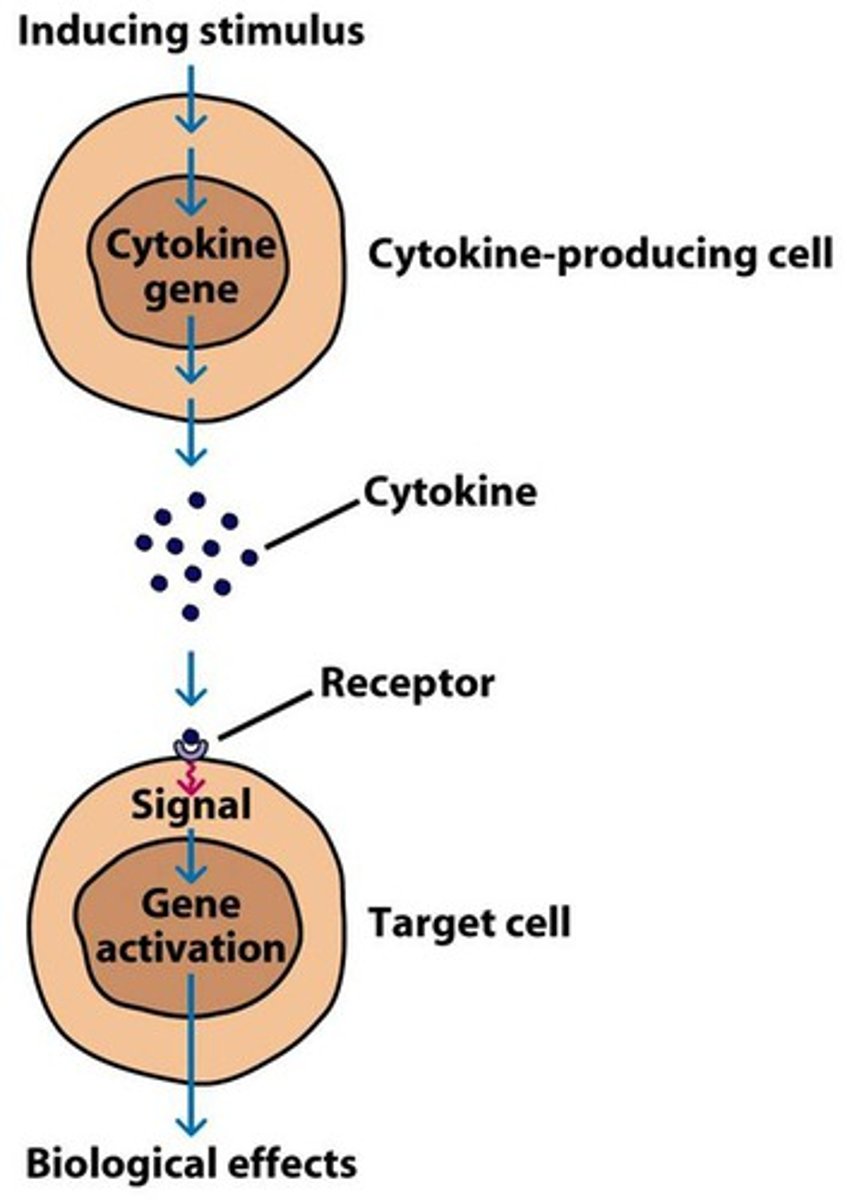
examples of cytokines
-erythropoietin (EPO): red blood cell production
-G-CSF & thrombopoietin: platelet production
-IL-2 and IL-4: T and B cell proliferation
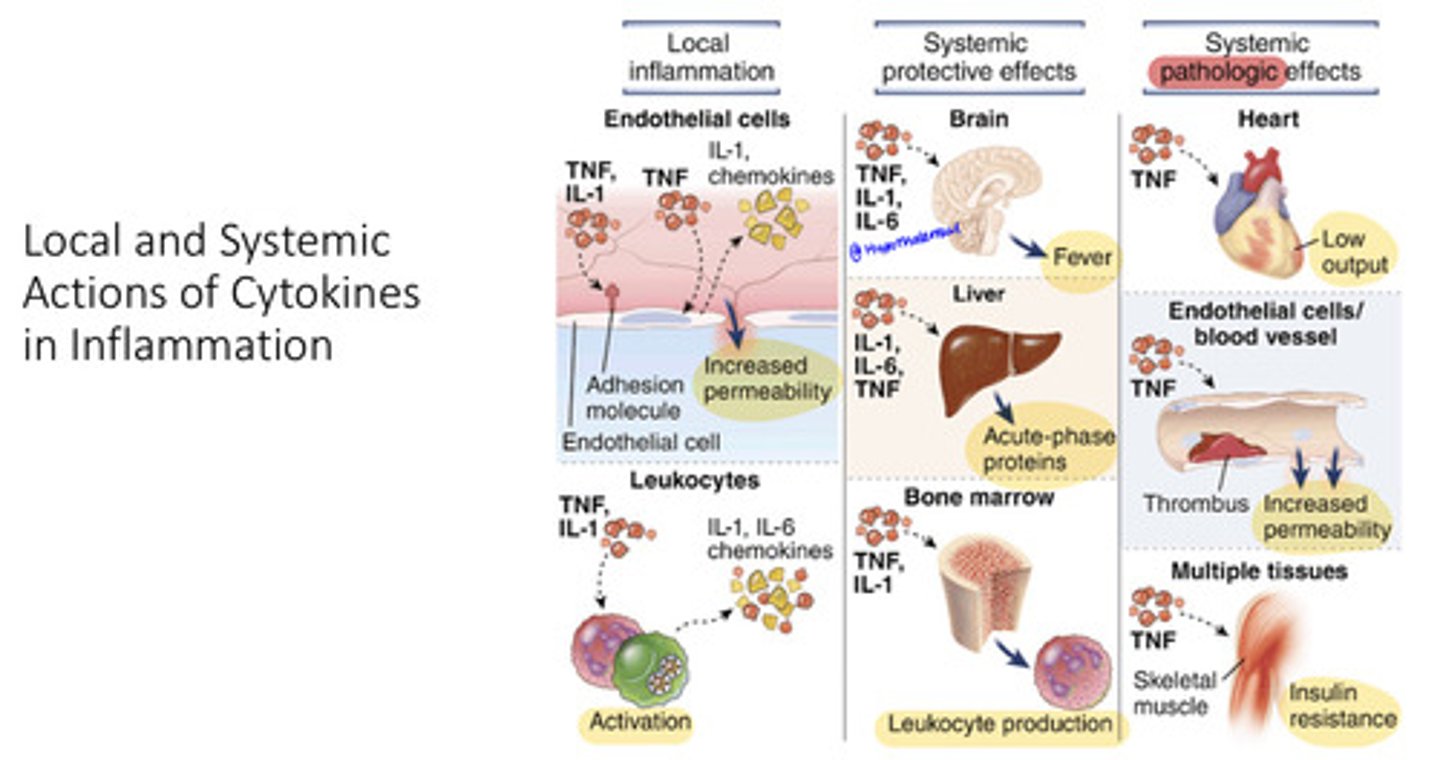
NFκB
a transcription factor that regulates the immune response and cellular stress, inducing the expression of over 150 genes, including cytokines, defense proteins (iNOS), and survival proteins that prevent apoptosis.
How do immune cells detect pathogens and respond?
Immune cells, such as macrophages, detect pathogens through receptors and release pro-inflammatory cytokines like TNFα and IL-1, initiating the inflammation response necessary for combating infections
What happens when TNFα and IL-1 bind to receptors on nearby immune cells?
these pro-inflammatory cytokines induce the NFκB pathways, resulting in increased cytokine production and enhanced immune activation
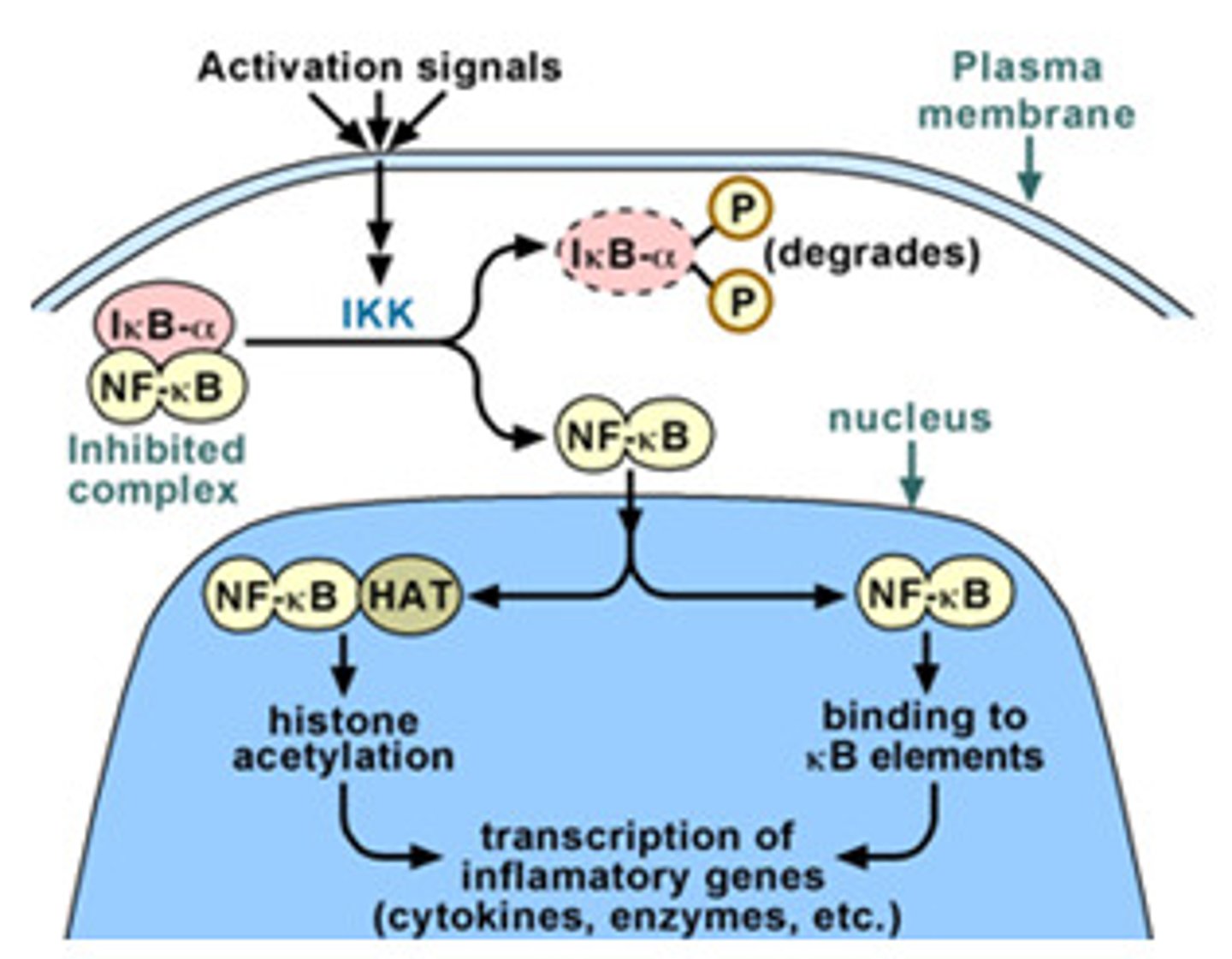
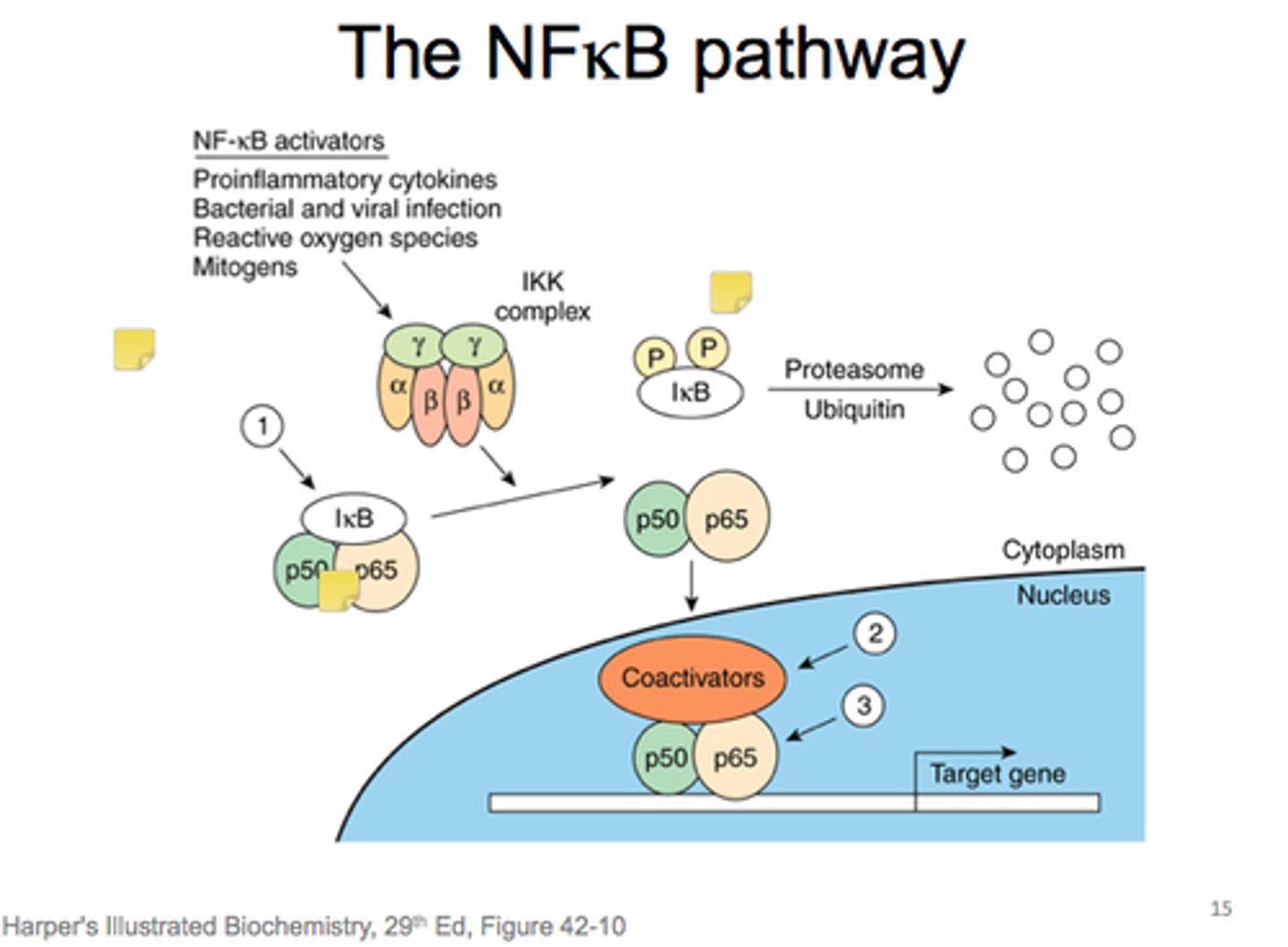
how is activation of NFκB different that other signaling discussed?
-different than other signaling that use phosphorylation
-activated by the degradation of the inhibitor IκB
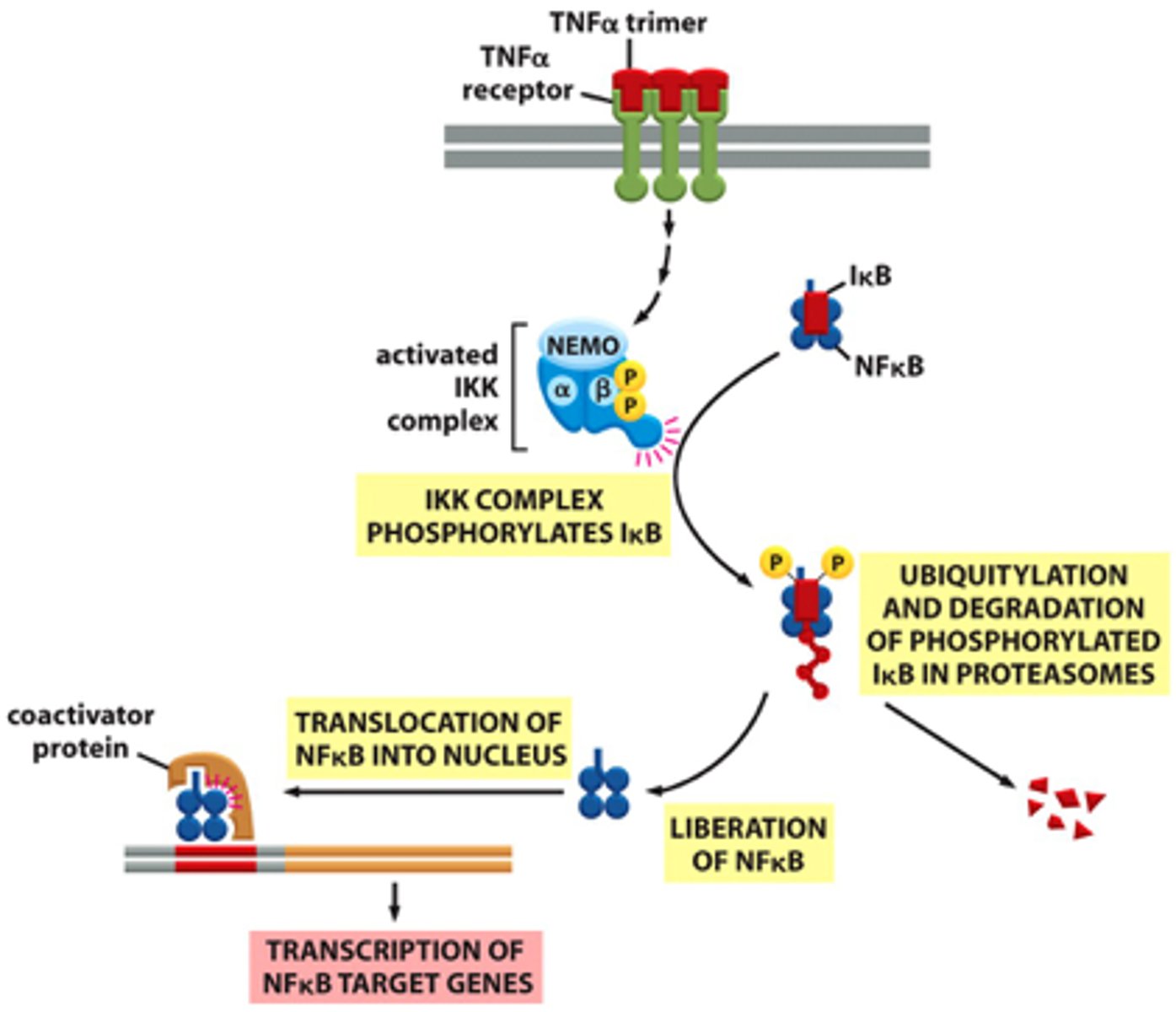
Overview of TNFα/NFκB signaling steps
1. TNFα or IL-1 cytokines bind to their receptors on immune cells, activating the kinase TAK1.
2. TAK1 phosphorylates and activates IκB kinase
3. IκB kinase phosphorylates IκB, which is bound to NFκB
4. E3 ubiquitin ligase recognizes the phosphorylated IκB and marks it for degradation by the proteasome
5. With IκB degraded, the NLS of NFκB is unmasked, allowing it to enter the nucleus
6. NFκB activates transcription of over 150 genes that promote survival and proliferation of immune cells.
7. Termination: Newly synthesized IκB binds to NFκB, returning it to the cytoplasm and shutting off the signaling pathway
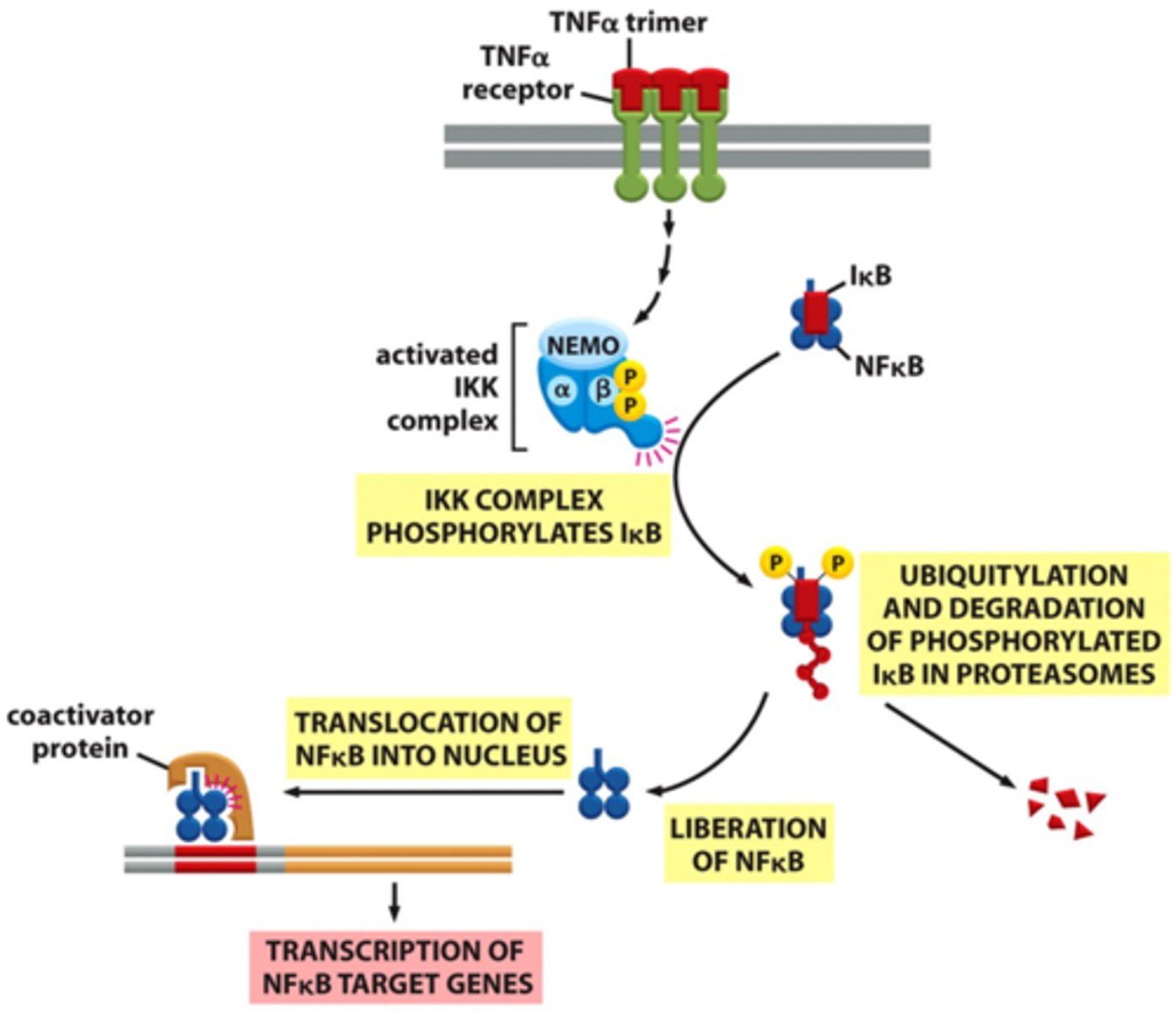
What triggers the activation of NFκB signaling pathway?
the binding of TNFα or IL-1 to their respective receptors which induces the activation of a kinase called TAK1.
What is the function of TAK1 in NFκB activation?
TAK1 phosphorylates and activates IκB kinase (IKK)
How does IκB kinase (IKK) contribute to NFκB activation?
IKK phosphorylates specific residues on the inhibitor IκB, which is bound to the NFκB dimer, leading to IκB degradation.
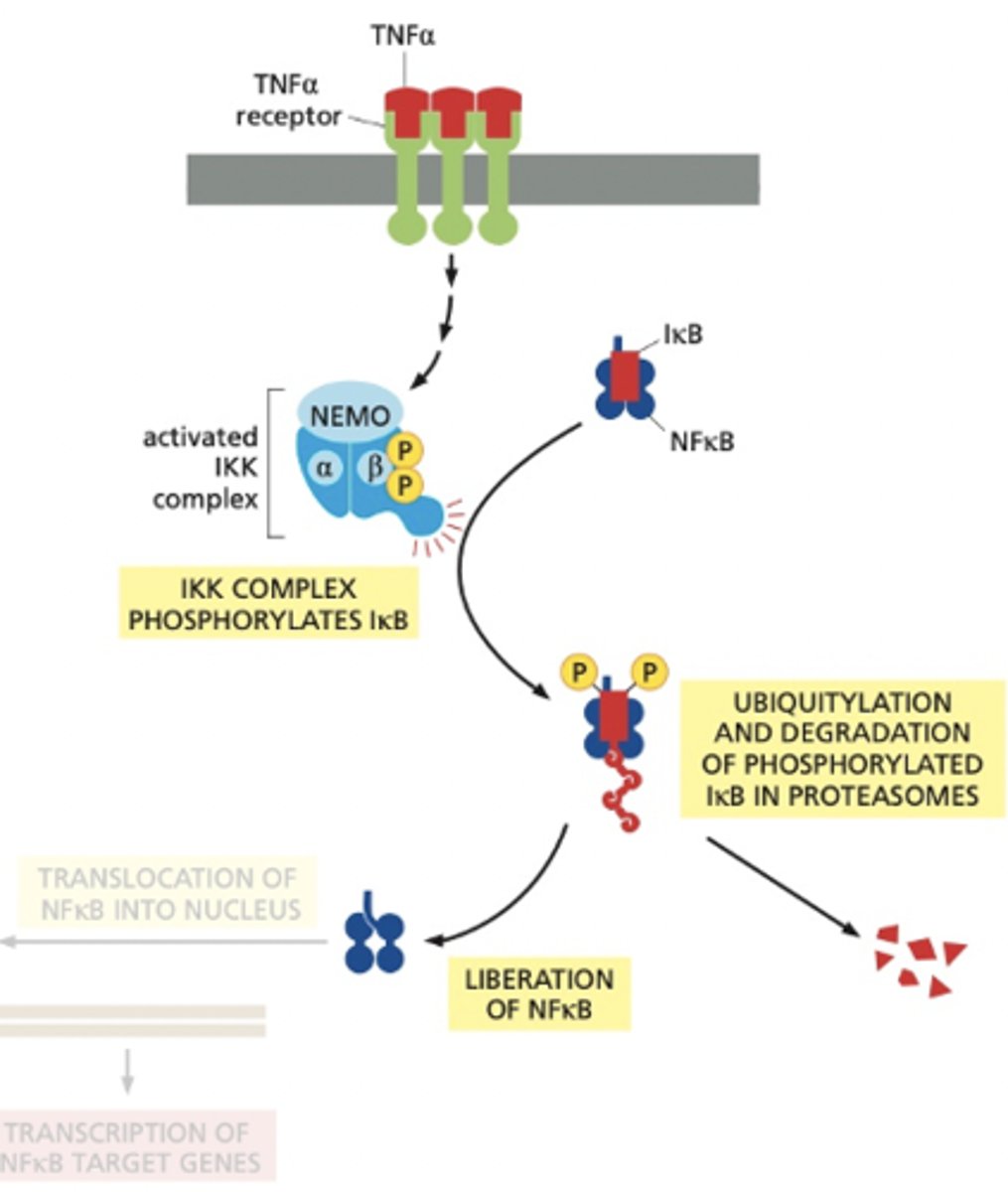
What happens to phosphorylated IκB?
Phosphorylated IκB serves as a binding site for an E3 ubiquitin ligase, which adds a polyubiquitin chain to IκB, targeting it for degradation by the proteasome
What occurs after IκB is degraded?
Flashcard 6: Shut-Off Mechanism
Q: A:
Once IκB is degraded, NFκB's nuclear localization signal (NLS) is exposed, allowing NFκB to translocate into the nucleus and activate the transcription of genes that promote survival and proliferation.
How is NFκB signaling terminated?
Newly synthesized IκB enters the nucleus, binds to NFκB, and brings it back into the cytoplasm, thereby terminating the inflammatory response after the pathogen is destroyed.