Orgo Exam 2 (17,18,19)
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
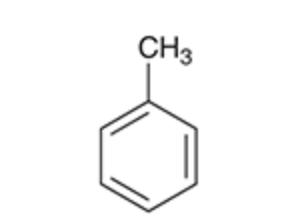
Toluene
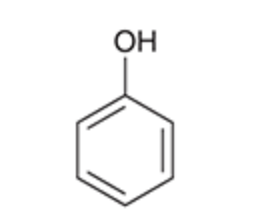
Phenol
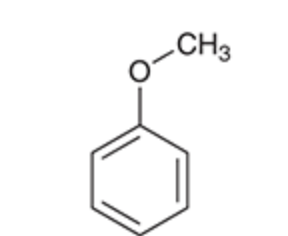
Anisole
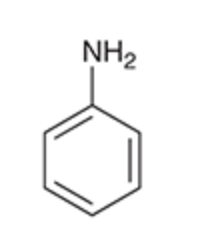
Aniline
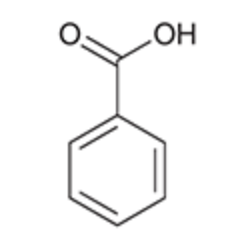
Benzoic Acid
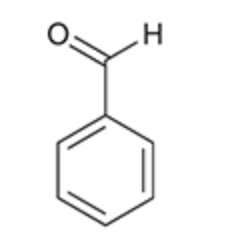
Benzaldehyde
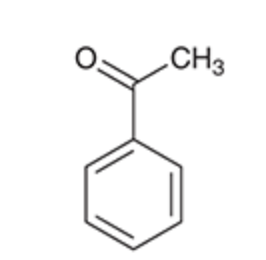
Acetophenone
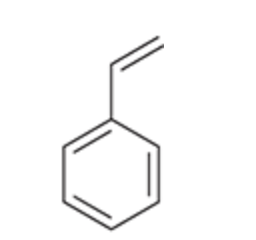
Styrene
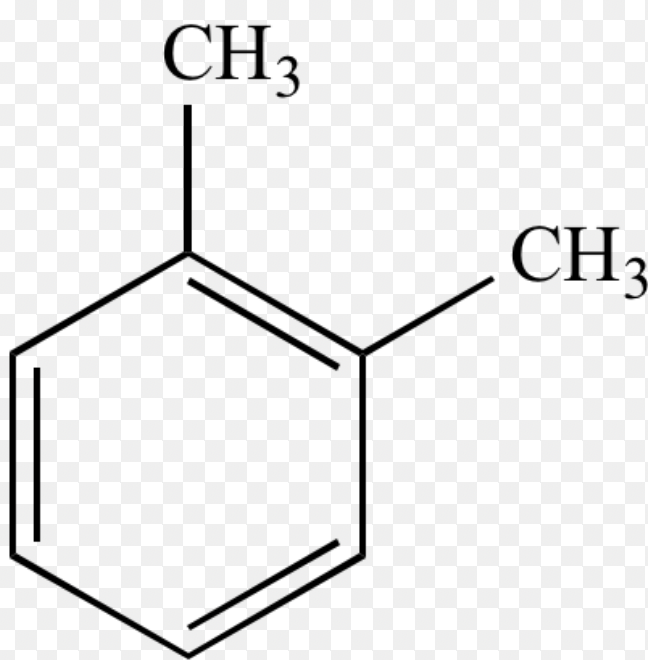
Xylene (Ortho)
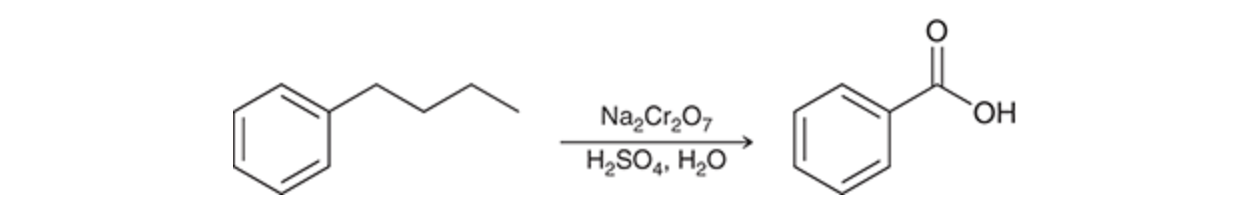
chromic acid reacting with alkylbenzene
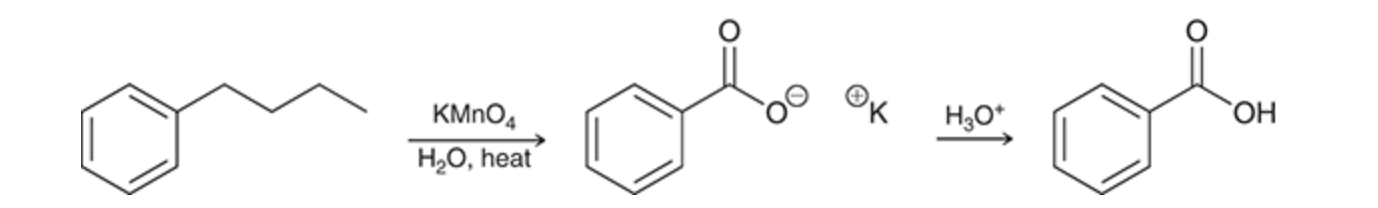
potassium permanganate (KMnO4), which must then be treated with a proton source in order to obtain benzoic acid.
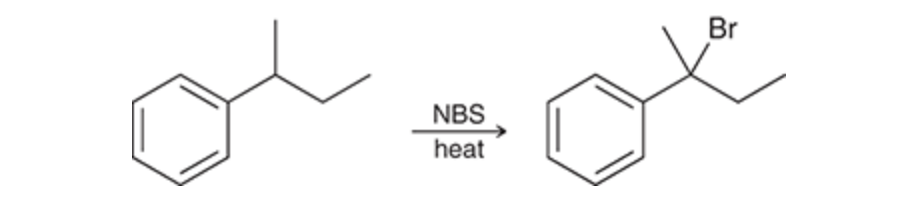
free-radical bromination also occurs readily at benzylic positions
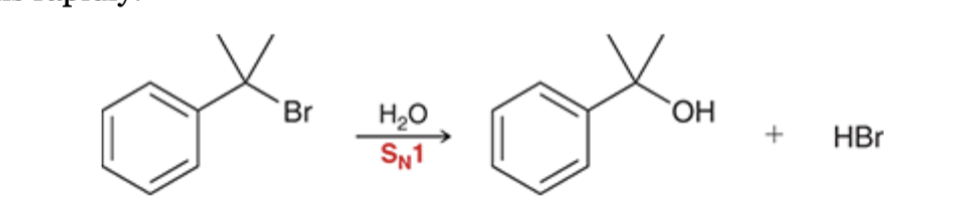
benzylic halides undergo SN1 reactions rapidly
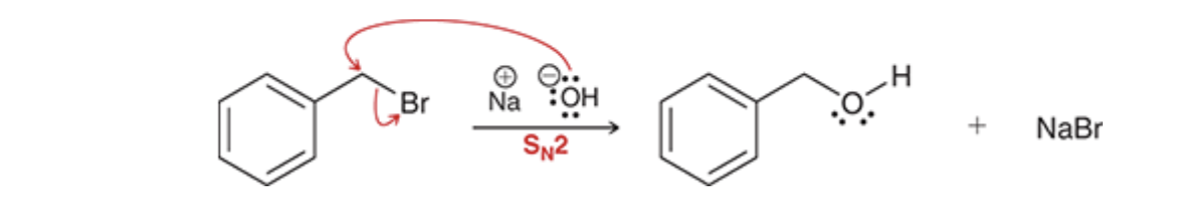
Benzylic halides also undergo SN2 reactions very rapidly
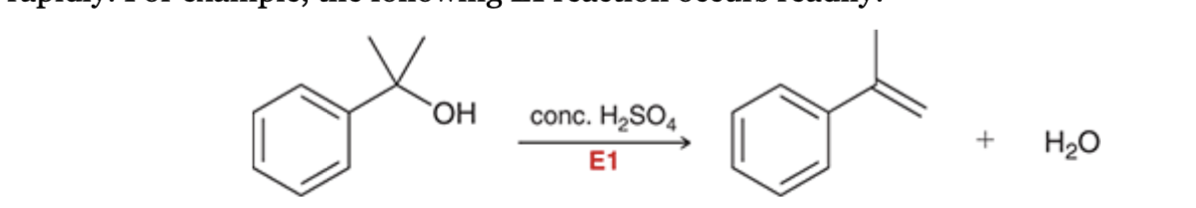
E1 reaction of benzylic halide
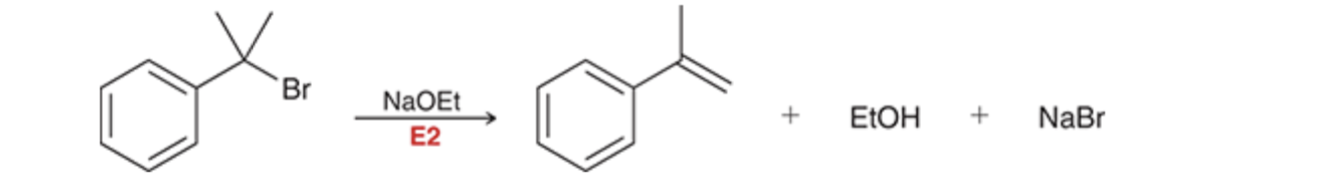
E2 reaction of benzylic halide
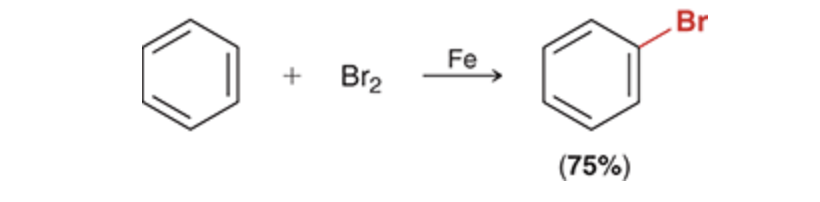
Bromination of Benzene
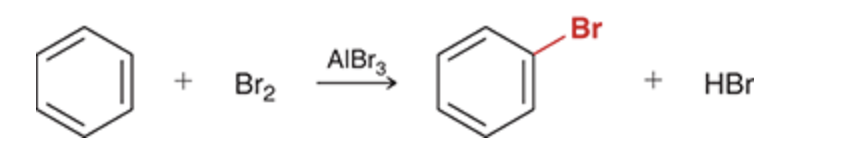
Bromination of Benzene
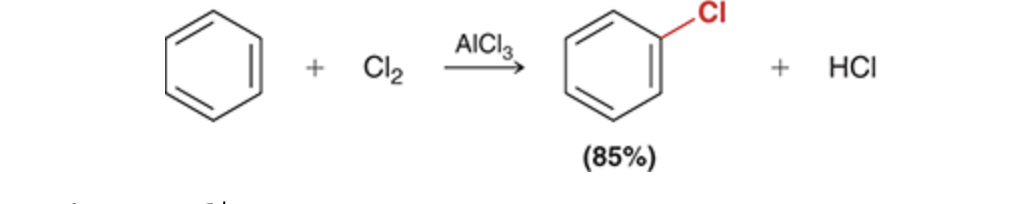
Chlorination of Benzene
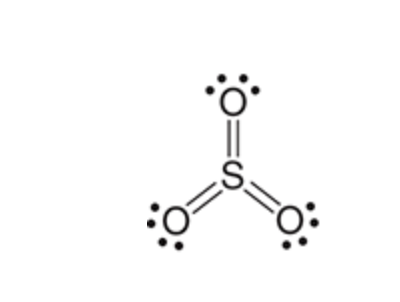
Fuming H2SO4
Sulfonation of Benzene
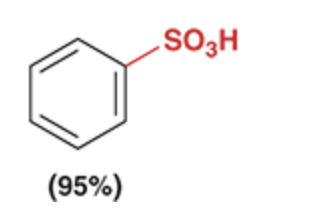
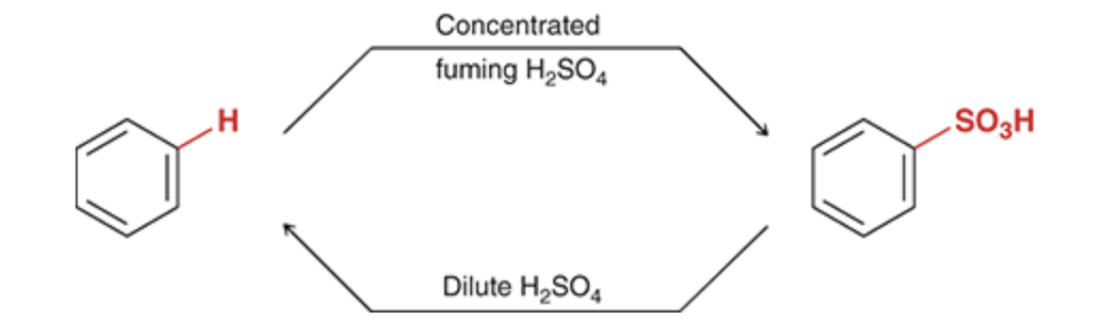
the reaction between benzene and SO3 is highly sensitive to the concentrations of the reagents and is, therefore, reversible
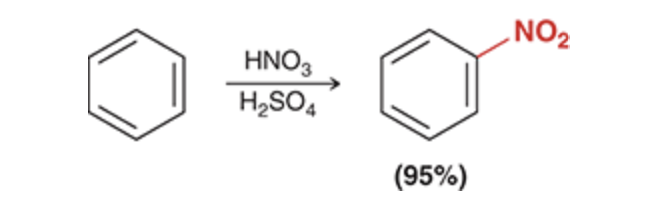
Nitration
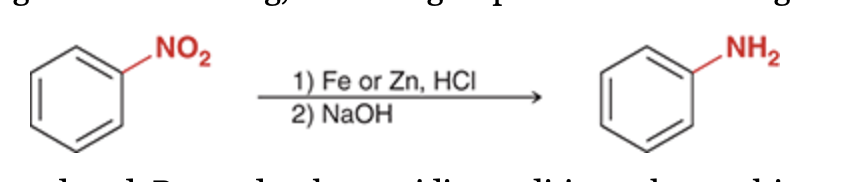
This method can be used to install a nitro group on an aromatic ring. Once on the ring, the nitro group can be reduced to give an amino group (NH2).
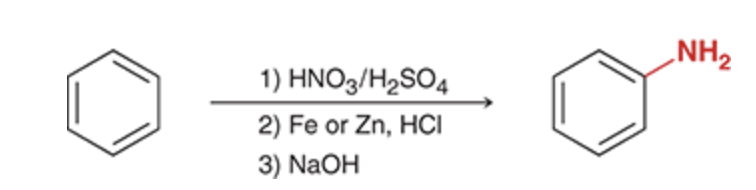
This process provides us with a general method for installing an amino group on a benzene ring. First, a nitro group is installed on the ring (nitration), and then the nitro group is reduced to an amino group
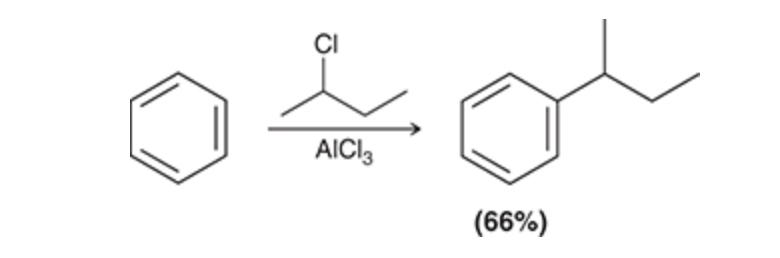
Friedel–Crafts Alkylation
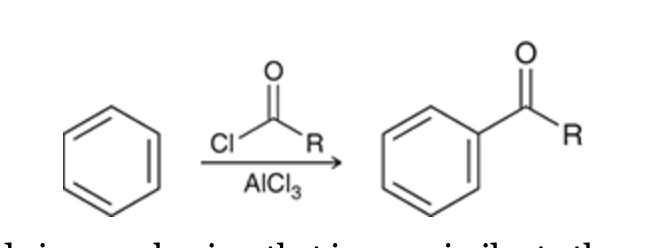
Friedel–Crafts Acylation
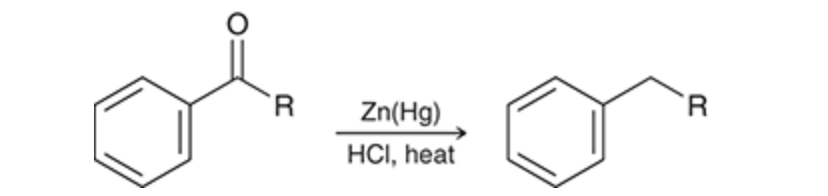
Clemmensen reduction
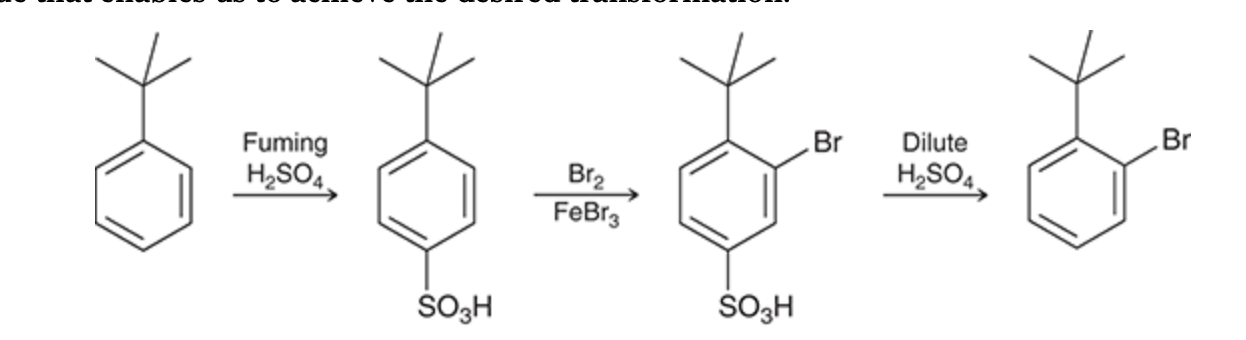
Blocking Group
Sulfonation is commonly used for this purpose, because the sulfonation process is reversible.
Bromination
Br2, FeBr3/AlBr3
Chlorination
Cl2, AlCl3
Iodination
I2, CuCl2
Nitration
HNO3, H2SO4
Sulfonation
Fuming H2SO4
Friedel-Crafts Alkylation
CH3(R)Cl, AlCl3
Friedal-Crafts Acylation
Cl-O=-R,AlCl3
Reduction (NO2 to NH2)
1)Fe or Zn, HCl 2)NaOH
Benzylic Bromination (CH3 to CBr3)
Excess NBS
Oxidation (CH3 to -=O-OH)
1) KMnO4, H2O,Heat 2) H3O+
Clemmensen Reduction(Just alkane)
Zn(Hg),HCl heat